pp. 43 & 44
Depending on which carboxylic acid derivative is present, the number of additions of the carbon organometallic species may differ. In the system below, a Grignard Reagent (phenyl magnesium bromide) is mixed with benzophenone. In this system, it does not matter if one equivalence of the Grignard Reagent is added, or if excess Grignard Reagent is added. There is only one carbonyl double bond present, and it is broken in the first step of the reaction. There is no other place for a Grignard Reagent to add and the C=O bond cannot reform as there are no good leaving groups present. The reaction will stop at this point and the alcohol will be formed upon addition of water during workup.
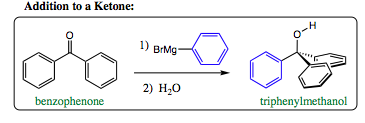
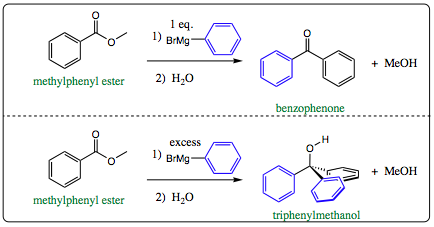
Use of an ester, however, introduces a bit of sophistication that must be taken into consideration. If one equivalence of Grignard Reagent is added to phenylmethyl ketone, benzophenone will be the product isolated. If excess Grignard Reagent is added, however, triphenylmethanol will be the isolated product.

Even one step further, the use of diethyl carbonate can give rise to 3 different products depending on how much Grignard Reagent added in the initial solution.
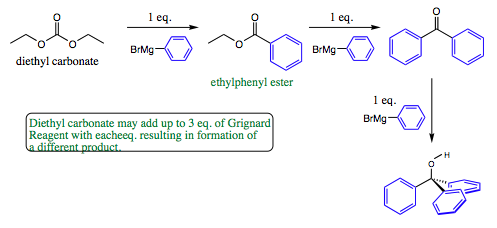
As seen from above, esters may may give either a ketone or an alcohol product, so care must be taken to add the correct amount of reagent to obtain the desired product.
Earlier, it was alluded to that both Grignard Reagents (RMgBr) and lithium reagents (RLi) could be used as nucleophiles, and that is true. In reality, however, there are some differences between these two organometallic species that we will need to address in order to ensure that you are able to pick the correct reagent (carbon nucleophile) for the system you are working with at the time.
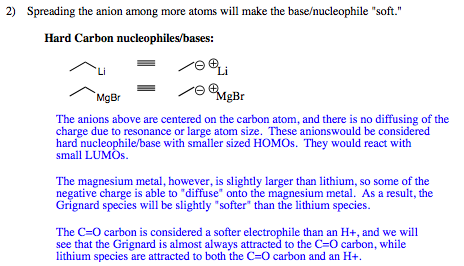
In order to understand the difference between Grignard Reagents and lithium reagents, we must revisit hard and soft charges that were originally covered in Chapter 3 pages 13-16.
Looking once again at hard versus soft charges:


| PREVIOUS PAGE (41 & 42) | Back to Index | NEXT PAGE (45 & 46) |