pp. 45 & 46
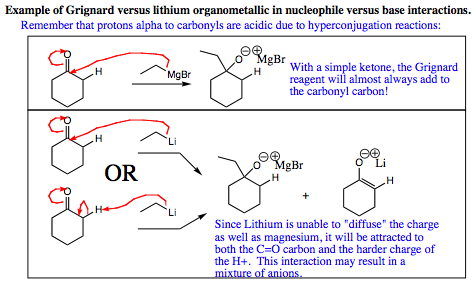
If you wish to have a nucleophilic addition occur, the safe bet is to go with the Grignard reagent. Lithium reagents are easy to form, and are nice to use as well, but the hardness of the charge can sway these species into acting as a base instead of a nucleophile in certain systems, giving a mixture of products.
Conjugate Carbon Nucleophilic Addition:
When a double bond is situated in a conjugated position next to a carbonyl group it is known as an a,b-unsaturated carbonyl. The presence of the conjugated double bond gives rise to a second possible electrophilic site on the molecule, thus, there are two potential products that may form. One product may arise from a 1,2-addition and the other product may arise from a 1,4-addition (also known as a Michael Addition). Resonance shows how the orbitals overlap and the electrons are all in conjugation, giving rise to resonance structures which place positive character not only on the carbonyl carbon, but also in the 4-position at the far end of the C=C bond.
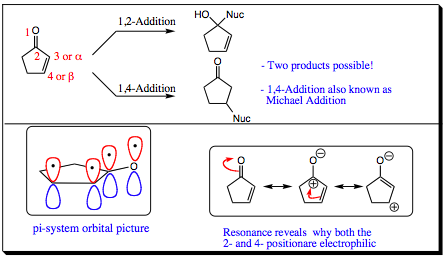
This resonance is further articulated using the following movie. Although the movie shows a triple bond in resonance with a carbonyl functional group, the electron movement will be identical to a system with a double bond conjugated to the carbonyl functional group. It is good to visualize and realize that similar systems all react, and interact, in similar fashions:
(For a larger version of the movie, please click here.)
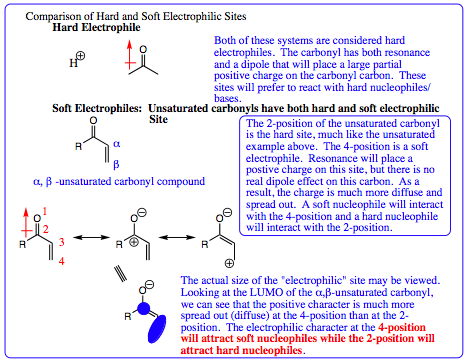
A comparison of hard and soft electrophilic sites may be accomplished in the same fashion as was done for nucleophilic species. A hard electrophilic site is one in which the positive charge is not spread out, but rather localized to a small area. A soft electrophile is one in which the positive charge is able to spread out over a larger area. I like to think of the hard charge as looking like a baseball (residing in a small area, hard and not easy to deform) while a soft charge is more like a beach ball (larger, but more able to be deformed or moved about). We are really chatting about orbital size. Remember in solubility that “Like dissolves Like.” In reactions, the same theory applies, but to orbital size. Orbitals of similar size will overlap much more efficiently and have stronger interactions. By looking at hard-hard and soft-soft charge overlap, it will be possible to predict when a 1,2-addition should occur and when a 1,4-addition should occur.

| PREVIOUS PAGE (43 & 44) | Back to Index | NEXT PAGE (47 & 48) |