pp. 41 & 42
Carbon Nucleophiles:
-Organometallic species are most often the type of carbon nucleophile that will be used. Remember that carbon is not very electronegative and has no lone pairs of electrons it its neutral state. Only the anion of a carbon atom will be nucleophilic.
The use of a metal counterion (forming an organometallic) helps stabilize the carbon anion (since carbon is not an electronegative atom, it does not hold a negative charge well), making it much easier to prepare, store, and use. The metal will also help stabilize the intermediate species that forms during the course of addition as well.
NaC¼N (Sodium Cyanide) is a very versatile nucleophile due to the presence of the CN triple bond. The cyano group has a negative charge on the carbon atom and will add to carbonyl functional group in the fashion shown below. Note that the mechanism of addition is identical to what has been seen with hydride addition, oxygen addition, and nitrogen addition to carbonyl groups. The negative carbon species acts as a nucleophile and attacks at the carbonyl center which carries a partial positive charge. Since carbon is less electrophilic than either nitrogen or oxygen, it will be least happy with a negative charge, and will, in general, be more reactive than either oxygen or nitrogen nucleophiles.
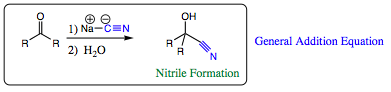
After addition of the cyano group, you may already have realized that the CN bond is polar and may also act as an electrophile. As such, the nitrile group is extremely versatile and may be converted quite easily to other functional groups as shown below:
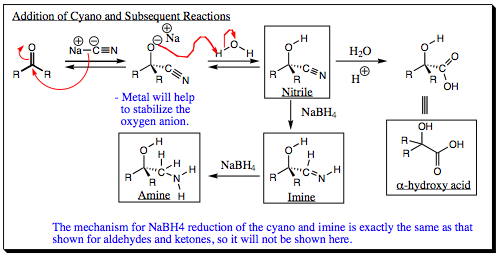
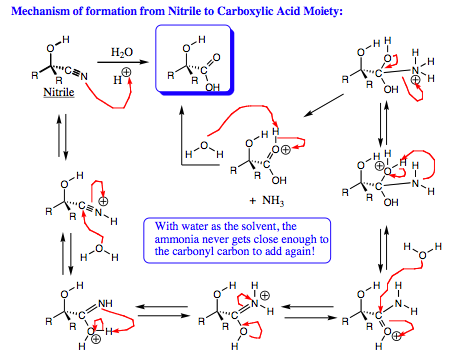
Several of these reactions we have already discussed. The example shown to the far right in which the alph-hydroxy acid is formed, this appears a little different from earlier systems. We have studied the addition of water to imines to form aldehydes and ketones, but why does a carboxylic acid form in this case? Note that the CN functional group has 3 formal bonds, 2 of which are pi-bonds. Since 2 pi-bonds are present, water may add twice, forming a carboxylic acid in lieu of an aldehyde or a ketone. The mechanism of formation for the carboxylic acid is shown below:
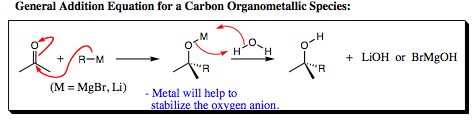
Grignard Reagents [R-MgBr] and other Organometallic Reagents [R-M] will comprise the largest portion of our carbon nucleophiles. Luckily, we have already seen these species in Chapter 3 when we first began to chat about nucleophilic species and their reactions.
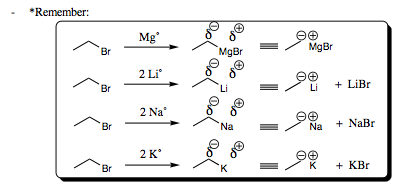
As was witnessed with sodium cyanide, the carbon atom of Grignard Reagents (where the counterion is MgX with X being Cl, Br, or I) or other organometallics will be anionic (carrying a negative charge). I will not draw out a long mechanism for this species, since it will follow the same mechanistic pathway as oxygen, nitrogen, halogen, and cyano nucleophiles already studied. I hope that you are beginning to see that all mechanistic pathways are generally the same, only the R-groups differ from system to system.
| PREVIOUS PAGE (39 & 40) | Back to Index | NEXT PAGE (43 & 44) |