pp. 13 & 14
Nucleophilicity versus Basicity:
As stated in the preceding pages, nucleophilicity increases down a column, while basicity does not. Why would this be so?
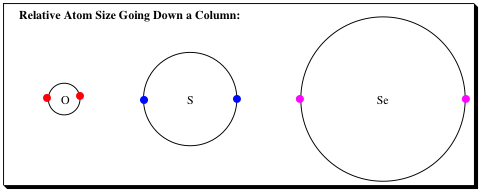
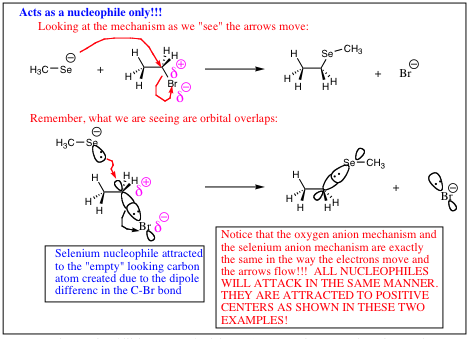
As you travel down a column, the valence electrons are held further from the nucleus in a larger orbital. The differential in orbital size as you travel down a column has the following effects: 1) There is less electron repulsion as the electrons are further apart from each other. 2) The electrons are not as strongly attracted to the nucleus as there is more shielding and the electrons are further from the nucleus. 3) The orbital is more easily mis-shaped, making it easier to "point" toward a carbon center (electrophile) allowing for bond formation to occur much more readily.
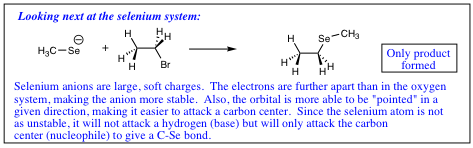
Think of the electron situation as hard and soft charge. The electrons around the oxygen are held in a small/tight space much like a baseball which is referred to as a “hard charge.” The electrons in selenium are held in a much larger space resembling a beachball. The electrons in the selenium orbital are much more spread out and are referred to as a “soft charge.”
A hard charge will be more reactive, and more willing to “rip” a proton from a molecule (act as a base) while a soft charge is not as reactive, and is more able to orient itself to form the more stable carbon bond (act as a nucleophile). The following adage tends to hold true;
![]()
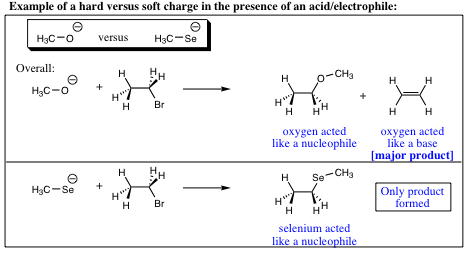
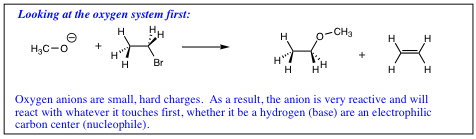
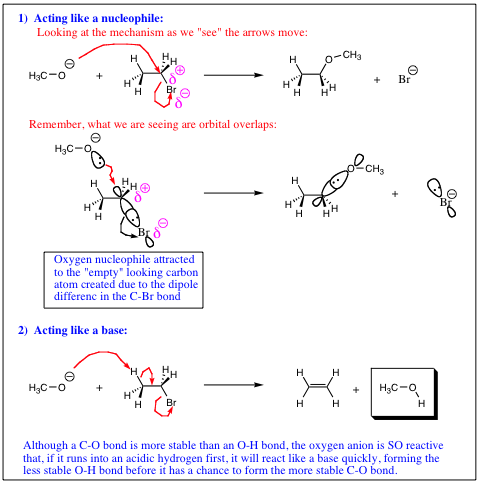
To summarize nucleophilicity versus basicity: 1) Less electronegative element in a row (containing lone pair of electrons) will be more nucleophilic. 2) Nucleophility will tend to increase down a column, while basicity will tend to decrease down a column.

| PREV. PAGE (11 & 12) | Back to Index | NEXT PAGE (15 & 16) |