pp. 7 & 8
Knowing that ethane has an energy different of 2.8 kcal/mole between the eclipsed and staggered conformation, it is possible to determine how much time the molecule spends in the staggered conformation with respect to the eclipsed conformation. The equations are followed as below:
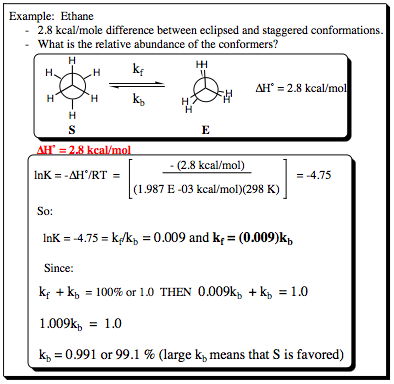
The Arrhenius equation allows us to determine the relative ratios of kf and kb based on the energy difference of the two conformers. With this ratio, knowing that they constitute 100% of the system population, it is possible to determine the overall ratios as shown above. This is a simple technique that yields an incredible amount of information. We will see more of this in the next section looking at cycloalkanes.
Cycloalkane Systems:
Most important factors giving rise to important conformations in acyclic systems will be important in cycloalkane systems as well. We are going to be studying mainly torsional and steric srain factors. Since we have looked at these factors earlier in the semester with respect to cycloalkane systems (Chapter 1), just a quick review of ring systems 3-5 will be given here, followed by a more in-depth study of the 6-membered ring system to follow.
1) Cyclopropane:
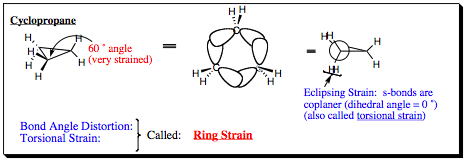
2) Cyclobutane and Cyclopentane:
Cyclobutane
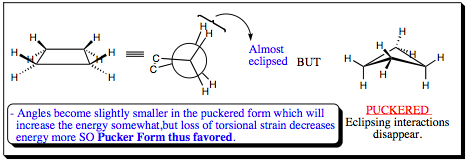
Cyclopentane: - Planer, no angle strain.
- Hypothesized by Baeyer to be nearly strain free (not the case)
- WHY NOT?

| PREVIOUS PAGE (5 & 6) | Back to Index | NEXT PAGE (9 & 10) |