pp. 5 & 6
A closer look at Gibbs free energy equation will give a better indication of what each portion of the equation entails and what it tells us about chemical systems and energies.
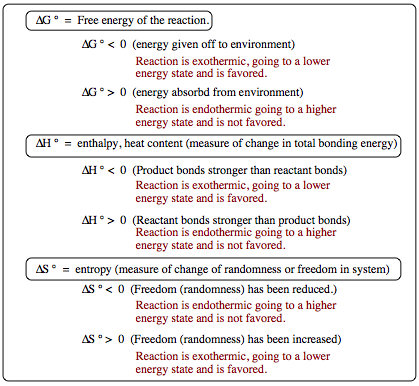
ÆG ¡ is the overall free energy of a system. Recall that nature is lazy and is always looking to find a lower energy state. If ÆG ¡ is less than zero, this means that energy was given off from the system to the surrounding environment, lowering the overall energy of the system. This type of change is exothermic (energy given off) and is favored. This does not tell us how fast a change will occur, but it does tell us whether or not a particular change is favored. ÆH ¡ is the overall change in bonding strength. When
ÆH ¡ is less than zero, this means that the product bonds are stronger than the bonds in the reactant(s). The energy of bonding is lowered, and the change will be favored. ÆS ¡ is the overall change in freedom or randomness of a system. Think about marbles constrained in a bag. If you pour those marbles onto a table, they are now free of the constraints of the bag and will roll everywhere given their new freedom. Trying to pick up each of the marbles and return them to the bag will be much more difficult and requires the input of a fair amount of energy (generally yours unless you can convince a younger sibling to do this work for you). Nature as a whole is moving toward more randomness (you can use this excuse the next time someone tells you to clean up your room). When ÆS ¡ is above zero, randomness and freedom have increased and the overall energy of the system has been lowered, which will be favored.
By using Gibbs Free Energy equation and looking at the Heat of Reaction it will be possible to more carefully study Conformational Isomers and determine when one will be favored over the other and to what percentage of the system it will make up overall.
Measuring Equilibrium and use of the Arrhenius Equation:
Use of simple equilibra ratios will allow determination of the time a molecule will theoretically spend in any given conformation within a system.
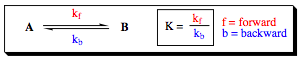
The equilibrium constant (K) is a measurable data point. Once it has been obtained for a particular system, its value may be used with the Arrhenius Equation to determine relative population states of conformers for a given system:
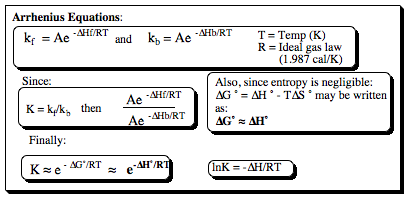
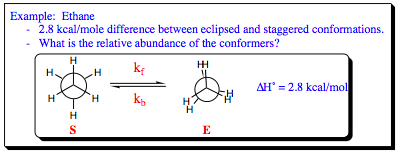
We can use this information in an examples to clarify better what it is we are trying to accomplish.
| PREVIOUS PAGE (3 & 4 ) | Back to Index | NEXT PAGE (7 & 8) |