pp. 25 & 26
Cycloalkane Systems:
Cycloalkanes are also called alicyclic compounds (aliphatic cycle). The discovery of cycloalkane molecules occurred over 100 years ago, and the field continues to grow at a very quick rate.
1) Cyclopropane:
Cyclopropane is the smallest known and most studied cyclopropane (Discovered by Freund in 1881). As with many discoveries in chemical fields, cyclopropane was discovered by accident. In research, we often find that nature's pathway is not always the one which we thought may occur. If the researcher is diligent, the unexpected twists which occur may lead to exciting new fields of study and do not need to be viewed as setbacks. As an old colleague once told me: "90% of what you try will not work and another 5% will not be interesting. Keep your eyes open for that final 5% and you will always be happy in your work."
In the first synthesis of cyclopropane, the goal of the original research was to form polyethylene, a long chain molecule with interesting properties. The hope was to mix 1,3-dibromopropane with 2 equivalents of sodium metal to form the product as shown in the equation below:


This reaction is an example of a metal insertion with subsequent attack onto an electrophilic center. In the original hypothesis, having a bromine on either end of the molecule would allow for the chain to continue to grow indefinitely, forming a long alkane chain with unique properties. What actually transpired is that the negative charge formed from metal insertion attacked the electrophilic center on the same molecule, causing a cycle to form as shown below:
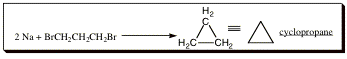
The mechanism is similar to that shown above, an is illustrated in the movie below:
|
|
For a larger version of the movie, please click here.)
Static mechanistic view of cyclopropane formation:

Expecting a solid from such a large acyclic chain, Freund had to have been quite surprised to obtain a viscous oil. Luckily for us, he was watching for that 5% of excitement (I am sure this must also have been accomplished on a Friday evening.), isolated and characterized the viscous oil, and he tapped into an entirely new are of chemistry of small ring alkane compounds!!

| PREV. PAGE (23 & 24) | Back to Index | NEXT PAGE (27 & 28) |