pp. 47 & 48
Before looking at actual transformations, let us take one more quick look at nucleophiles. The Grignard was shown to be a slightly softer anionic charge than a lithium reagent due to the relative size of magnesium and lithium. A magnesium cation can share more of the negative charge with a carbon anion versus the lithium cation. In terms of 1,2- versus 1,4- addition pathways, however, both nucleophiles are considered hard. These are not the only two types of carbon nucleophiles, so we must introduce a few more, shown below:
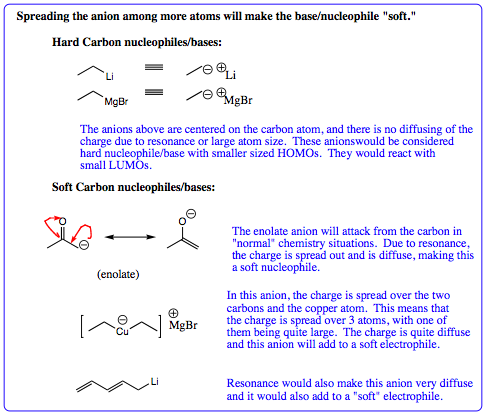
As seen from this information, both Grignard reagents and lithium reagents are considered hard nucleophilic charges and would be expected to add 1,2- with a carbonyl. The lithium reagent charge is sometimes hard enough that it acts as a base, rather than a nucleophile. As the anionic charge becomes more spread out, the charge becomes softer, and it would be expected that the nucleophile would be more inclined to add at the 1,4-carbonyl position. This is, in fact, what is witnessed. Anions with resonance, such as enolates (shown above) have negative charges spread out, are considered soft, and will generally add to the 1,4 – carbonyl position. Copper intermediates have charge spread over 2 carbon atoms and the large copper atom. These organometallic species are also considered soft and are also expected to add at the 1,4-carbonyl position. In general, most anions with good resonance, or the copper organometallic intermediate may be expected to add in a 1,4-fashion. Carbon anions without resonance will be expected to add at the 1,2-position.
Copper species are formed by attacking CuI or CuCl with a Grignard reagent or a lithium reagent as shown below:
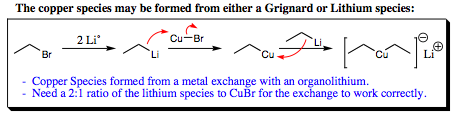
The copper salt may be present in stoichiometric amounts, or as a catalyst. If the copper salt is used in catalytic amounts, it is continually regenerated by the lithium reagent (or Grignard Reagent) as it reacts with the carbonyl system. As one of the copper intermediates shifts a substituent, it is attacked again by another lithium reagent (or Grignard) to reform the reactive species.
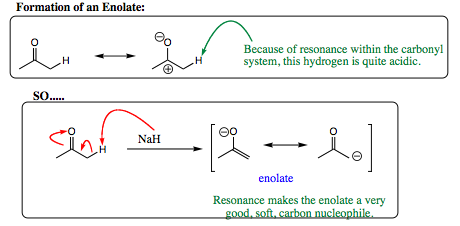
an enolate is formed by removal of a hydrogen from the position adjacent (alpha) to the carbonyl center. Generally a sterically bulky lithium base is used, or a very small, hard, hydride such as NaH. An example of an enolate formation is shown below:
Here are some examples of species that add 1,2- and species that add 1,4- to unsaturated carbonyl species.
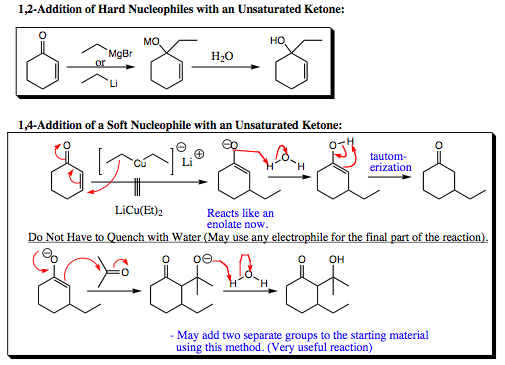
Notice that quenching the intermediate of a 1,4-addition reforms the carbonyl functional group. Since the enolate is a carbon nucleophile, it is also possible to quench the reaction with a different electrophile such as acetone shown on the bottom. This possibility gives the 1,4-addition reaction a tremendous amount of versatility and makes it a powerful synthetic tool.
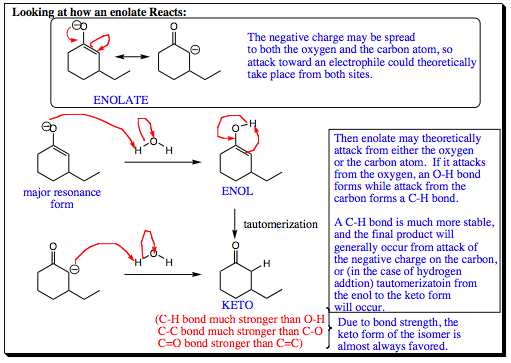
When an enolate is quenched with water, you have a small electrophile (the hydrogen in water). We would expect that the small electrophile would add to the hard nucleohphile site of the carbonyl system, which would be the oxygen atom. Since oxygen is more capable of holding a charge than carbon, much of the negative charge of an enolate is centered in a small space around the oxygen atom. Indeed, the hydrogen atom originally adds to the oxygen, forming an ENOL that reverts back to the ketone by a process called TAUTOMERIZATION.
| PREVIOUS PAGE (45 & 46) | Back to Index | NEXT PAGE (49 & 50) |