pp. 49 & 50
Summary of 1,2- vs. 1,4-Addtion Reactions

The best has been saved for last, and we now embark upon the final carbonyl reaction of the semester!! Our final reaction this semester is going to be…
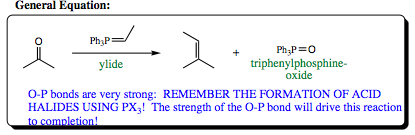
THE WITTIG REACTION: The Wittig reaction forms a double bond by reaction of a ketone/aldehyde with a phospho-ylide via the general equation shown below:
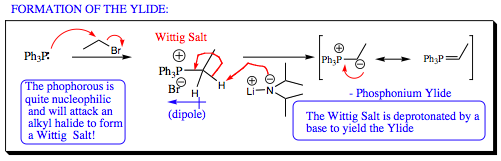
The first step in a Wittig Reaction is formation of a Wittig Salt by reaction of triphenylphosphine (or triethylphosphine or other types of tri-substituted phosphines) with an alkyl halide as shown. The Wittig Salt is generally very stable and may be stored for extended periods of time before use.
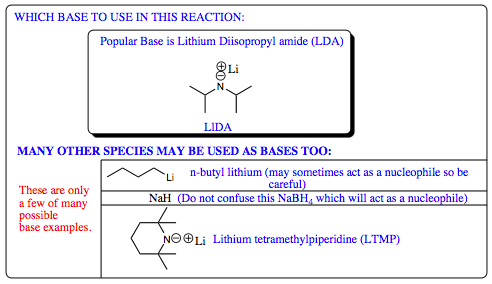
The second step in the Wittig Reaction is formation of the ylide which is a carbon nucleophile stabilized by the presence of a phosphorus cation on an adjacent atom allowing for resonance stabilization of the carbon anion. Many different bases have been shown to work in this step from NaOH to lithium diisopropyl amide (LDA). The type of substituents attached to the starting materials will most likely dictate what type of solvent, and hence what type of base will be used.
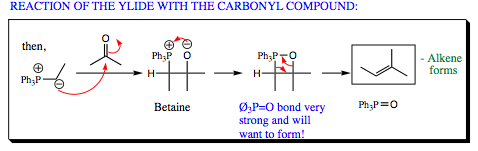
The third step in the Wittig Reaction is mixture of the ylide with a ketone/aldehyde as shown below. The carbon nucleophile will attack the carbonyl carbon as we have seen countless times this chapter, forming a Betaine. The oxygen anion will then attack the phosphorous cation. P-O bonds are very strong so this step is quite exothermic even though a 4-membered ring has been formed. In the final step, phosphorous and oxygen will form another bond as oxygen breaks the C-O bond and phosphorous donates the electrons from the P-C bond into the carbon system forming the final C=C bond seen in the product. The side product, triphenylphospine oxide, may be easily removed via column chromatography giving the alkene product in good yield.
As we learned earlier, double bonded molecules may exist as Geometric Isomers. In the following Wittig Reaction, there are two possible isomers which may form, cis- and trans-. The cis- isomer has previously been shown to be higher in energy, and we would expect the trans-isomer to predominate. This is not the case. The higher energy cis- isomer is favored, which may initially be confusing. A closer look at this system, however, will clarify items for us.
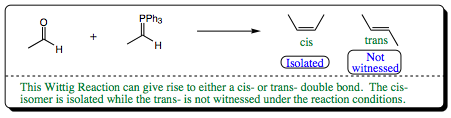
As we follow the mechanism, it will be noted that stereochemistry of product formation is set in the initial C-C bond formation (formation of the Betaine). As such, we need to take a closer look at this step to determine how the stereochemistry is set and why the cis- conformation is favored:
| PREVIOUS PAGE (47 & 48) | Back to Index | NEXT PAGE (51 & 52) |