pp. 19 & 20
Resonance picture of carboxylic acid derivatives.
Carboxylic Acid functional groups themselves present a unique problem, the presence of both a carbonyl bond and an OH bond. Due to the presence of the hydrogen on the oxygen attached to the carbonyl carbon, the system is extremely acidic (Chapter 3).
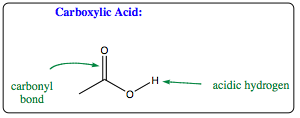
How does this acidic hydrogen alter reactivity of this system, and is it still possible to reduce a carboxylic acid functional group?
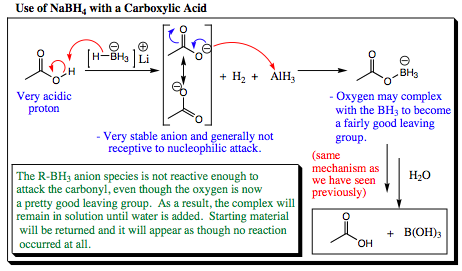
When sodium borohydride is mixed with a carboxylic acid, it acts as a base to remove the acidic hydrogen as shown below. The resulting species has an oxygen boron bond, and will remain in solution as a fairly stable intermediate. Sodium borohydride is not nucleophilic enough to attack at the carbonyl center, so no further reaction may occur. When water is added at the end of this reaction sequence, the carboxylic acid will be reformed. Many chemists have been fooled by, and frustrated with, this reaction as they witness hydrogen gas being released in step one, but isolate only starting carboxylic acid upon work up.
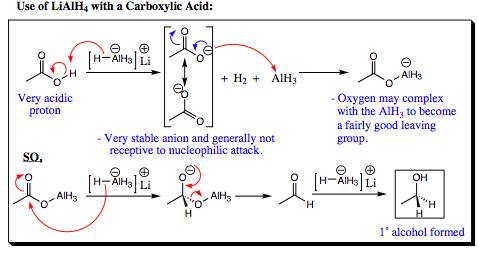
Mixing lithium aluminum hydride with a carboxylic acid results in a first step identical to that discussed above. The oxygen anion will then attack the neutral aluminum atom to give the oxygen – aluminum bond shown below. In this case, however, lithium aluminum hydride is reactive enough to attack the carbonyl carbon of the oxy-aluminum carbonyl complex, kicking off the oxygen aluminum species and forming an aldehyde. The aldehyde is further reduced as discussed previously to give a 1° alcohol after workup.
Atoms Other Than Hydrides as Nucleophiles (Carbonyl Reduction Reaction):
The last several pages have been spent studying addition reactions of a hydride to carbonyl groups. Although a hydride is the most simple nucleophile, it is not the only nucleophile that may be used for this purpose. There are literally thousands of nucleophiles that may be used to attack a carbonyl center. We do not have time to cover all of them (and I know this greatly pains you), but we can highlight the important groups and, even more important, reveal that if you understand the mechanism behind how these nucleophiles add, you will also understand how all the other thousands of nucleophiles will also add to carbonyl systems. Let us begin our trip upon the nucleophile highway.
Halides as Nucleophiles:
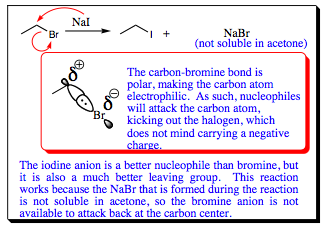
In Chapter 3 it was noted that C-X (X = Cl, Br, I) bonds are good “latent electrophiles.” These bonds are polar and halogens are stable anions due to their electronegativity, thus making good leaving groups. Since halogens may carry a negative charge and have multiple lone pairs of electrons, they are considered good nucleophiles. In fact, we are going to spend a good portion of a future chapter discussing their nucleophilic tendencies toward alky halides. As good as halogen anions are at displacing alkyl halides, however, they are not good nucleophiles for carbonyl systems. Let us take a quick look at why this is so.
But,
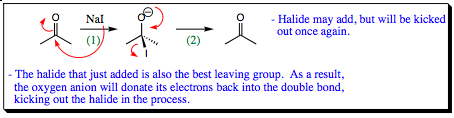
As seen, halogens will quickly add to a carbonyl carbon in step (1), but they are the best leaving group attached to the central. As a result, they will be “kicked-off” the molecule in step (2) as quickly as they add. No halogens may be added to a carbonyl in this fashion. If you wish to add a halogen to a carbonyl compound (forming an ACID HALIDE), the methods on the next page must be followed.
| PREVIOUS PAGE (17 & 18) | Back to Index | NEXT PAGE (21 & 22) |