pp. 21 & 22
Use of PX3 and SOX2 to add a Halogen to a Carbonyl Functional Group:
Use of PX3, where X = Cl, Br is a traditional method for adding a halogen to a carbonyl group. In this case, the starting material is a carboxylic acid. The general transformation is shown below:
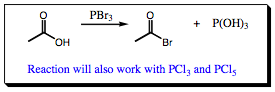
Walking through the mechanism uncovers that the arrow flow is not much different than what was witnessed for sodium borohydride and lithium aluminum hydride reactions. The phosphorous atom is electron deficient due to the 3 halogen atoms that are attached. Attack on the central phosphorous atom occurs as a result and is followed by subsequent halide loss. The halogen anion deprotonates the carboxylic acid species, leaving a neutral oxa-phosphorous intermediate (A). Since the phosphorous atom still has two halogen atoms attached, it will be attacked twice more by carboxylic acid starting compounds to give (B). At this point in the reaction, phosphorous has 3 oxygen atoms attached instead of the 3 halogens. As a result, the phosphorous atom is no longer electron deficient, but is slightly electron dense, allowing it to remove a proton from the H-halogen species formed in the reaction mixture. The halogen anion, once again liberated, now attacks at the carbonyl carbon, kicking out a good oxygen-phosphorous leaving group and forming the final acid halide product. This step is repeated twice more as shown below to complete the reaction sequence. Acid Halides are important functional groups as they are the most reactive of all carboxylic acid species and will interact with the widest variety of nucleophiles. Having a simple, efficient method of formation for these functional groups helps to simplify organic synthetic transformations to a great extent as we will see in upcoming chapters.

Thionyl halides are also very popular in the formation of acid halides from carboxylic acids. The mechanism of this reaction is very similar to that followed above and is left to students to work through for fun and relaxation.

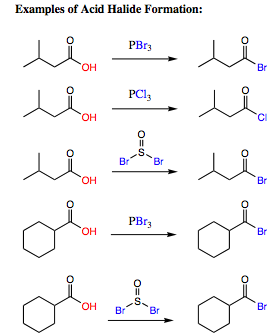
Some examples of Acid Halide Formation are shown below:
| PREVIOUS PAGE (19 & 20) | Back to Index | NEXT PAGE (23 & 24) |