pp. 17 & 18
Reduction of Amides
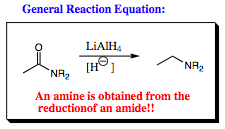
It may be expected, based on the ester reduction, that mixing LiAlH4 with an amide functional group would result in a 1¡ alcohol. If this was your first thought, congratulations, you are beginning to extrapolate your knowledge to unknown systems based on the chemistry you know. Although a good hypothesis, your initial thought would not be correct in this case. The reduction of an amide by LiAlH4 gives an amine. Let us look at the mechanism of this reaction for an explanation as to how this transformation occurs.
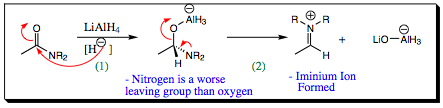
Initial
attack by the hydride occurs in exact fashion as witnessed in previous
examples. The second step,
however, is what sets amide reduction apart from ester reduction. In this case, after the oxygen atom has
attacked the neutral aluminum center, we would generally expect the oxygen to donate its lone pair back into
the carbon center, reforming the pi-bond as was witnessed with esters. We have to recall which atom, nitrogen
or oxygen is less electronegative, and which atom is most likely to donate a
lone pair of electrons. A nitrogen
atom will donate a lone pair of electrons more readily and quickly than an
oxygen atom, kicking out the oxygen atom which is bound to aluminum and thus
becoming a pretty good leaving group. An iminium ion is formed instead of an aldehyde!!!
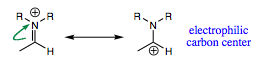
The iminium ion is also a good electrophile due to polarity and resonance. LiAlH4 will attack the carbonyl carbon of the iminium ion in the same fashion as previously studied carbonyl functional groups.
Since a nitrogen atom was attached to the carbonyl carbon in lieu of an oxygen atom, the final product of this reduction reaction is an amine, not an alcohol.
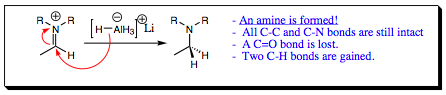
It is important to note that the mechanism for an aldehyde, ketone ,ester, and amide reduction are all very much the same. The difference in isolated products has to do with the R-group attached to the carbonyl carbon. With esters and amides, the attached group contains a heteroatom making a good leaving group. As a result, the original molecule is reduced twice and not just one time. In all cases either an alcohol or an amine is formed, the pi-bond being totally reduced by hydride ions.
Summarizing Hydride Reactions with Carbonyl Compounds:

A. NaBH4
Sodium borohydride will reduce both aldehydes and ketones. Due to electrophilicity differences, an aldehyde may be reduced in the presence of a ketone if desired. Reactions run in alcohol solvents generally proceed at an accelerated rate due to solvent participation, although initial reagent solubility must be taken into consideration. Sodium borohydride is not reactive enough to reduce carboxylic acid derivatives such as esters or amides.
B. LiAlH4
Lithium aluminum hydride is more reactive than sodium borohydride. One consequence of the extra reactivity is that LiAlH4 will reduce aldehydes and ketones at the same rate with no selectivity realized. The extra reactivity also means that LiAlH4 will act as a base in the presence of a protic solvent. As a result, alcohol solvents, which help increase the rate of sodium borohydride reductions, cannot be used with lithium aluminum hydride. The extra reactivity does mean that lithium aluminum hydride will reduce carboxylic acid derivatives such as esters and amides. In the case of esters, a 1¡ alcohol is the isolated product and in the case of an amide, an amine is the isolated product. Although these products appear much different from each other, the mechanism for formation is similar in each case and the student is reminded to have confidence the mechanistic theory and arrow flow of these reactions.
| PREVIOUS PAGE (15 & 16) | Back to Index | NEXT PAGE (19 & 20) |