pp. 15 & 16
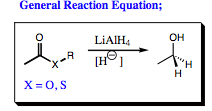
Lithium aluminum hydride (LiAlH4) has much in common with sodium borohydride. Both of the central atoms have 3 substituents attached to them in their neutral state with one empty p-orbital left over (sp2-hybridized). Both of the reagents will “give” or “kick-off” a hydride ion to regain neutrality, thus acting as nucleophiles. Both will reduce aldehydes and ketones to alcohols in very good yields.
The reagents differ by the central atom and counterion. With an aluminum center and a lithium counterion, lithium aluminum hydride has much greater reactivity than does sodium borohydride. As a result of this difference, although their mechanism of reactivity is similar, there are some important differences which we will discuss.
One of the differences noted is that LiAlH4 will reduce aldehdes and ketones at the same rate. Due to its higher reactivity relative to sodium borohydride, lithium aluminum hydride reduces aldehydes and ketones at such a quick rate that there is no selectivity witnessed. In the system below, recall that sodium borohydride could reduce an aldehyde in the presence of a ketone, and selectivity could be realized in the product. Use of lithium aluminum hydride, however, will result in isolation of only the diol with both aldehyde and ketone functional groups reduced. You will wish to keep in mind this selectivity difference if attempting a reduction of an aldehyde in the presence of a ketone if you do not wish to reduce both functional groups.
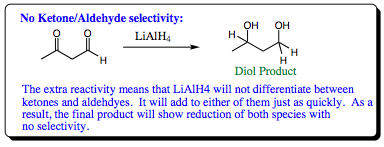
The extra reactivity of lithium aluminum hydride also renders it basic toward alcohol solvents leading to decomposition of the reaction mixture as well as the formation of side products, and lowering of overall yields of product.
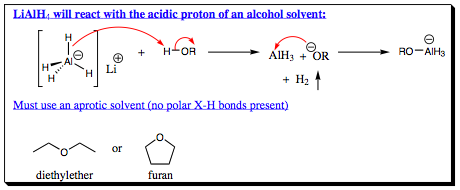
As such, deprotonation of an alcoholic solvent may occur, creating a new nucleophilic species in solution. As a result, only aprotic solvents may be used when LiAlH4 is the reducing agent, and the reaction must be quenched (carefully) with water at the culmination of reduction.
Reduction of Carboxylic Acid Derivatives with Lithium Aluminum Hydride:
The extra reactivity does allow lithium aluminum hydride to reduce carboxylic acid derivatives. We will look at several of these transformations in the following pages.
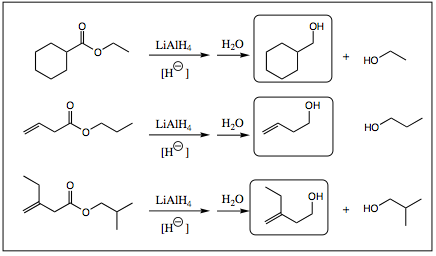
As seen above, the reduction of an ester results in the formation of a 1° alcohol. At first glance this may seem a bit confusing, but a more careful scrutiny of the system, and mechanism of reaction, will reveal that this product should be expected.
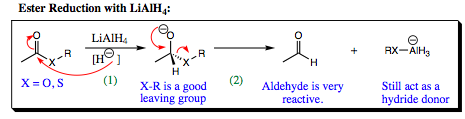
The first step in ester reduction is attack of the hydride at the carbonyl carbon, breaking the pi-bond to form an anionic intermediate. This step is identical to that seen in redcuction of aldehydes and ketones, however, the heteroatom connected to the carbonyl carbon of a carboxylic acid derivative makes for a good leaving group. The pi-bond is reformed, kicking out –XR as a leaving group and forming an aldehyde. As we have seen previously, aldehydes are more reactive than esters and LiAlH4 attacks the aldehyde functional group as it forms (step 3 shown below). A 1° alcohol is, thus, formed. Notice that an aluminate intermediate is formed in similar fashion to the boronate that was formed earlier.
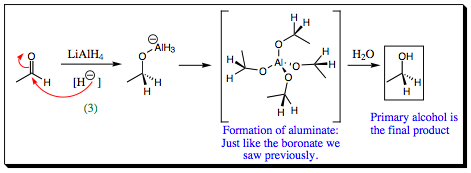
Notice that it does not matter what R-group is attached to the oxygen atom, the final product is always a 1° alcohol. The oxygen attached to the carbonyl is always removed with its R-group attached. This secondary product is generally water soluble and may be removed from solution during workup of the reaction.
| PREVIOUS PAGE (13 & 14) | Back to Index | NEXT PAGE (17 & 18) |