pp. 13 & 14
Relative Rates of Aldehyde and Ketone Reduction using Sodium Borohydride:
When using sodium borohydride, the following rate pattern may be noted:
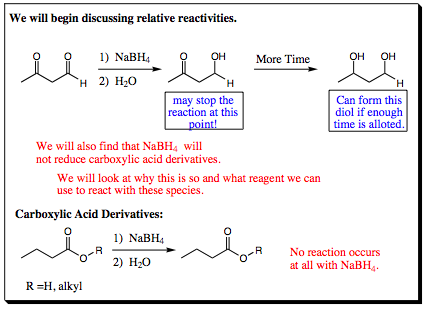
In the system above, it is shown that sodium borohydride will react with an aldehyde much more quickly than it will react with a ketone. In fact, in many systems it is possible to reduce an aldehyde in the presence of a ketone. Since sodium borohydride must have the same reactivity in each case, we must be seeing a difference in reactivity between the aldehyde and ketone functional groups. Why will sodium borohydride reduce an aldehyde faster than it will reduce a ketone, and why will sodium borohydride not reduce a carboxylic acid derivative at all? What we are looking at is the size of the partial positive character located on the carbonyl carbon. The larger the partial positive character, the more electrophilic the carbon center will be and the faster a hydride may attack the group. NaBH4 is drawn to the larger partial positive charge of an aldehyde faster than the slightly smaller partial positive charge of a ketone (due to hyperconjugative interactions). With carboxylic acid derivatives, resonance donation from the oxygen connected to the carbonyl carbon reduces partial positive character even more, reducing electrophilic character and reactivity with sodium borohydride.

The movie below explains the differing electrophilicities of the carbonyl functional groups for you in more detail:
(For a larger version of this movie, please click here)
Use of LiAlH4 (Lithium Aluminum Hydride) in Carbonyl Reduction Reactions:
As witnessed in the preceding pages, sodium borohydride is a nice reagent for use in reducing aldehydes and ketones. In fact, sodium borohydride is able to reduce aldehyde functional groups in the precence of ketones owing to their differing electrophilic properties. It was also determined that sodium borohydride did not have the nucleophilic power required to reduce carboxylic acid derivatives. As such, a more nucleophilic hydride donor is required to enable the reduction of carboxylic acid derivatives.

| PREVIOUS PAGE (11 & 12) | Back to Index | NEXT PAGE (15 & 16) |