pp. 9 & 10
Electrophile:
The term electrophile is also derived from the Greek language. Electros (electron) and philos (loving) combine to give us this little bit of nomenclature. Remembering that an electron is negatively charge, an electrophile must be positive in character. As such, an electrophile can be a cation or a “hidden cation.” A hidden cation is one in which either resonance, or a dipole moment, induces charge separation placing positive character on an atom that may then act as an electrophile. Some examples are shown below:
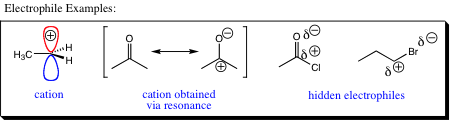
As nucleophiles are related to Lewis Bases, electrophiles are related to Lewis Acids, both areas of which will be covered later in this chapter.
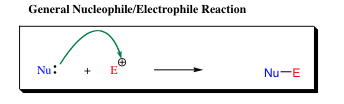
Nucleophilicy and Electrophilicity:
The terms Nucleophile and Electrophile are used specifically when bonds to carbon are involved. Acid and base terms are generally used when hydrogen transfer is involved.
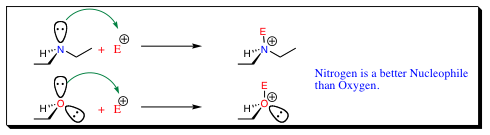
Nucleophilicity:
Nucleophilicity deals with the tendency for a system to donate electrons and will follow base trends to a degree. Nucleophilicity trends may be categorized in three main areas: 1) Less electronegative elements containing a lone pair of electrons are more likely to donate that lone pair of electrons than a more electronegative element with a lone pair of electrons (ie N > O and P > S). As will be seen, basicity will also folow this trend when dealing with atoms
containing heteroatoms in the same row. 2) Atoms further down a column will be more nucleophilic. 3) Anionic atoms will be more nucleophilic than the neutral species (Anionic atoms will also be more basic than the neutral species). Examples highlighting the three main areas are shown in the table below:
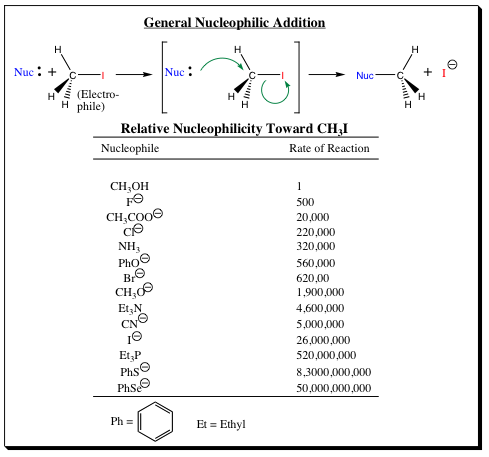
As the table reveals, an oxygen anion (CH3O-) is more nucleophilic than a neutral oxygen (CH3OH). Selenium, which is in the same column as oxygen, but located in the 4th row compared to oxygen being located in the 2nd row, is 2.6 X 104 times more nucleophilic than oxygen. When viewing atoms in the same row, it is noted that nitrogen is approximately 4.0 X 104 times more nucleophilic than oxygen.
| PREV. PAGE (7 & 8) | Back to Index | NEXT PAGE (11 & 12) |