pp. 7 & 8
Molecules and solvents that lack a polar X-H bond are referred to as aprotic. This type of bond has a very wide range, and may be polar or non-polar. As such, there are a wide variety of solvents available to the organic chemist in setting up systems for study and manipulation. The fun is just getting started. Listed below is a sampling of some of the more common aprotic solvents.
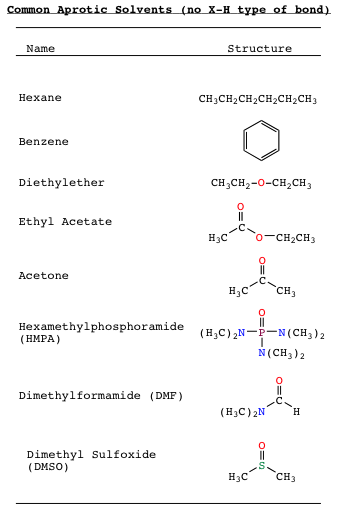
Note that aprotic solvents may contain heteroatoms, and may be quite polar (contain a large Dipole Moment). Aprotic solvents may not, however, contain a polar X-H bond. Since aprotic solvents may be polar, if they are mixed with a protic molecule, or solvent, hydrogen bonding between between the polar aprotic systems and protic systems may occur as shown below:
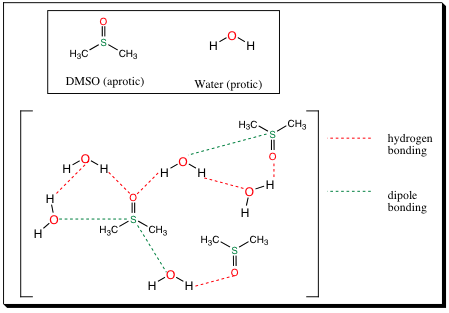
Notice that the oxygen in DMSO may hydrogen bond with the hydrogen in water. This is a favorable interaction. Also notice that the oxygen in water, which is partially negative due to water’s Dipole Moment, will interact with the sulfur atom in DMSO due to DMSO’s dipole moment and resonance which makes the oxygen in DMSO electron dense and the sulfur electropositive. These two molecules have good interactions with each other, and indeed, DMSO is miscible in water solvent and vice versa.
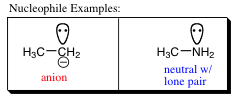
Nucleophiles and Electrophiles:
We have seen how charge separation may effect physical characteristics such as boiling point and solvation characteristics, We will now explore how charge separation helps determine chemical activity as well. Organic Chemistry is governed by a simple axiom of the flow of electrons from an electron source to an electron sink, in order to fill valence shells and create more stable bonds. Organic Chemists have given names to these electron sources and sinks, which we will now study.

Nucleophile:
The term nucleophile is derived from the Greek “nucleo” meaning nucleus, and “philos” meaning loving. It is truly a nucleus lover. Remember that the nucleus is positive. Thus, a nucleophile would be a molecule that is electron rich. An electron rich molecule, or center, could be an anion or a lone pair (in a neutral molecule), such as the two examples shown below.
As we shall see, the characteristics that make a Nucleophile are also the same characteristics that make a Lewis Base. There is much overlap in Organic Chemistry. If you can keep in mind that electrons always flow from a source to a sink, it will be easy to see the where and why these overlaps occur. It will also become easy to follow the electron flow that occurs during these processes.
| PREV. PAGE (5 & 6) | Back to Index | NEXT PAGE (9 & 10) |