pp. 11 & 12
For more subtle systems, other factors must be taken into consideration. In the example below, the nucleophiles are both carbon anions. Here we have the same atom, and the same charge. How is it determined which is the better nucleophile? The only difference with each of these anions is the double bond present in the lower molecule. One rule of thumb is that the less stable system will be more reactive, and will react more quickly. This particular case shows the effects of resonance, stability, and reactivity.
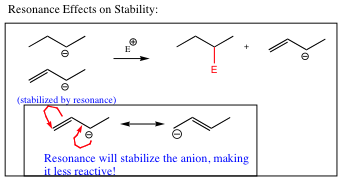
Resonance spreads out electric charge, stabilizing both cations and anions. As such, the conjugated system above will be less reactive, and the alkyl system, in which the electron charge is not spread out, but is centered all on one atom, will attack the electrophile first. We will find, however, that the extra reactivity of the top system will also make it more basic than the conjugated anion, and we will deal with how to determine the best pathway in subsequent chapters. At this beginning stage, let us stick with the total amount of charge on the reacting atom.
Systems may become even more subtle, but no more difficult to determine. Let us look at one further example. In the system below, resonance is not present in either molecule, and the anion is situated on a carbon in both cases.
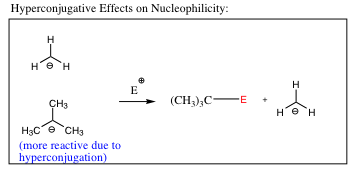
Why is the t-butyl anion more reactive? For this explanation, we need to think back to double bond stability (link back to that movie) and hyperconjugation. It was stated that C-H bonds situated adjacent to the double bond (p-orbitals) helped to stabilize the double bond by donating electron density. In the t-butyl anion above, the orbital containing the lone pair of electrons has 3 adjacent C-H bonds, each of which may donate electron density. As such, the C-H bonds add electron density to an already negative center, making it more negative, and more reactive. The top anion (methyl anion) does not have any C-H bonds that are able to donate electron density, so it will be less reactive. The movie below highlights hyperconjugation in anions (and cations) and explains how it makes anions more reactive and cations less reactive.
(For a larger version of the movie, please click here)
For a final review and pictorial description of nucleophile/electrophile interactions up to this point, please view the movie below:
(For a larger version of this movie, please click here.)
| PREV. PAGE (9 & 10) | Back to Index | NEXT PAGE (13 & 14) |