pp. 23 & 24
Nomenclature for Alkanes:
We are quickly reaching a point where it is difficult to identify all of the molecules we are drawing by site alone (and we are just getting started). Early in the history of organic chemistry, there were few known molecules, and those that were discovered were named often on a whim. Morphine, which we discussed earlier, was named for the Greek God of Dreams (Morpheus). Urea is also a unique name,
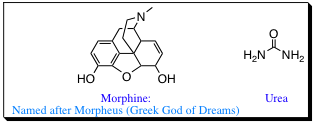
and there are many examples of molecules with unique names in organic chemistry. As more and more organic molecules were discovered, it become necessary to find a method for naming that was simple enough for everyone to follow the rules. With over 1 million molecules known, and the number increasing daily, some type of Universal Nomenclature is needed (Can you imagine having to memorize all the names of each compound!).
The method followed is from the International Union of Pure and Applied Chemistry (IUPAC). IUPAC nomenclature identifies compounds by two main areas: 1) Root - Establishing the number of carbons in the longest chain, and 2) Suffix - Describing the type of bonding in the molecule.
For sp3-carbon compounds - Alkanes - straight chain, the following rules apply:
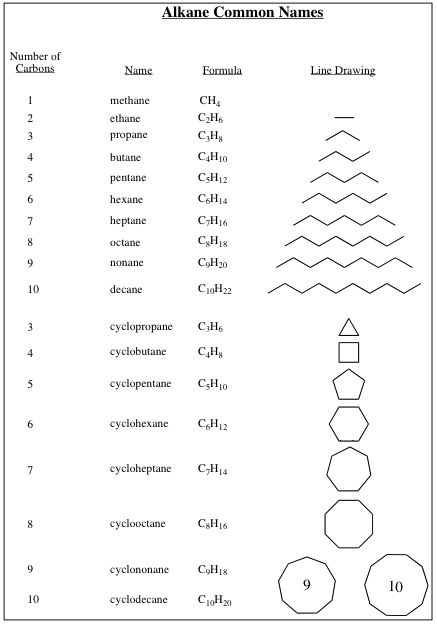
| 1) | Molecule | that contains only sp3-hybridized carbon atoms is designated with an ending -ane. |
| 2) | Molecule | is named as from table (Common Alkane Names) below: |
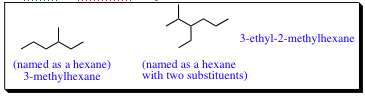
| - For sp3 | -carbon compounds that contain branching: | |
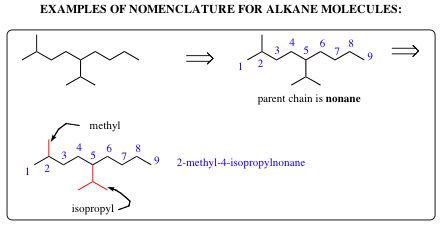
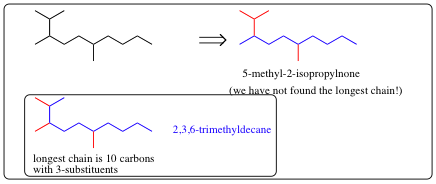
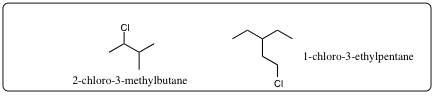
| a) | Find the longest continuous carbon chain present (Parent Chain and Name) | |
| b) | If two diferent chains are present, select the one with the larger number of branch points. | |
| c) | Number the atoms in the Parent Chain. | |
| d) | Number the substituents. | |
| e) | List the substituents in alphatical order. |
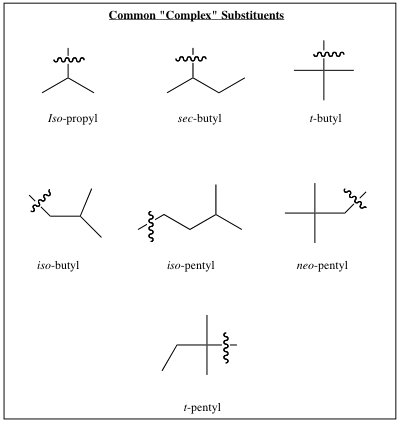
A table of common substituents is listed below:
| PREV. PAGE (21 & 22) | Back to Index | NEXT PAGE (25 & 26) |