pp. 21-22
Formula of Acyclic Alkanes:
When dealing with hydrocarbons, it is very time consuming to add up each atom for each molecule that you are to draw. It would be much easier if there were a formula that could be used to determine how many hydrogens are present when you draw a hydrocarbon with a certain number of carbons, especially if you are making good use of the line drawing model. For acyclic alkanes containing only single bonds (what are known as saturated molecules as they have the maximum number of possible hydrogen atoms) the formula is CnH2n+2. Every new C-C bond requires the addition of 2 hydrogens.
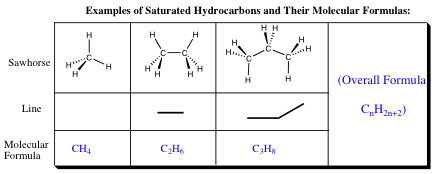
As long as your molecule adheres to the rule of being a saturated, acyclic hydrocarbon, you may always determine the number of carbons and hydrogens by using the molecular formula above.
Structural Isomers:
Now that we can construct molecules, and draw them using several different models, another problem may arise. We also now know how to determine a molecular formula, but is the molecular formula unique for each particular molecule or may there be more than one representation of a given molecular formula? When drawing molecules, you will quickly discover that each molecule does not have a unique molecular formula. You will quickly begin to draw Structural Isomers, which are molecules with the same molecular formula but different connectivity (Different backbones). To ease confusion, Structural Isomers also go by the alias of Constitutional Isomer. Let us take a look at the formation of a Structural or Constitutional Isomer.
Begin with a hydrocarbon containing 3-carbons. There is only one way to connect this particular molecule, forming only one structural isomer. Rotation about the bond does not lead to another structural isomer, but a Conformational Isomer. Since single bonds are able to rotate without breaking the molecule, and they rotate quickly at room temperature, we do not worry about Conformational Isomers (Conformers) at this stage. To this 3-carbon molecule, we add another methyl group (a hydrogen must be removed in the process).
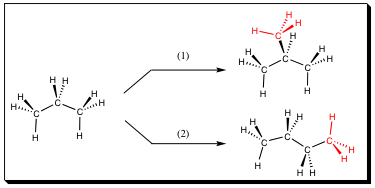
The methyl group may be added to the middle of our 3-carbon molecule (pathway 1) or to the end of our 3-carbon molecule (pathway 2). Both new compounds formed have only single (sigma) bonds and adhere to the CnH2n+2 formula (C4H10). It is quickly noted, however, that the sequence of bonding is quite different in the new molecules. We have created a structural isomer! For C4H10, there are two structural isomers, but the number of isomers will vary depending on the number of carbon atoms in a particular system. A short list is noted below:
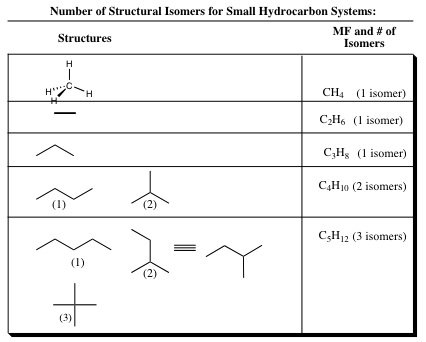
The number of isomers rises quickly as the number of atoms increases. The alkane C40H82 has more than 62 trillion isomers! If you can write them all out by the end of this semester, you will receive an automatic A. The must be written out in pencil!!

With the advent of isomers, there are now several types of carbon atoms noted. Not all carbons contain the same number of hydrogens connected to them. As with different isomers, there has to be a method of determining how many hydrogens, or non-hydrogen groups, are attached to a particular carbon. Using the isomers from C5H12, it can be shown how to differentiate between carbons with differing groups attached. A primary carbon (1¡) is a carbon in which there are four groups attached, only one of which is not hydrogen.
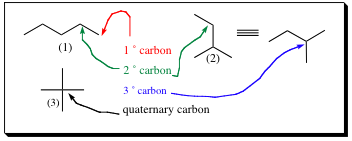
A secondary (2 ¡) carbon is one in which four groups are attached, two of them being non-hydrogens. A tertiary (3 ¡) carbon is one in which there are four groups attached, three of which are non-hydrogen, and a quaternary carbon is one in which four groups are attached, non of which are hydrogen atoms.
| PREV. PAGE (19 & 20) | Back to Index | NEXT PAGE (23 & 24) |