pp. 17 & 18
Although the transition state of a Cope rearrangement has aromatic character, this particular reaction requires a great deal of heat to proceed. One of the reasons is that a sigma bond does have to be severed along the pathway. Even with this difficulty, the Cope rearrangement has found wide use in organic synthesis. As the example below shows, it can be readily used to form larger ring systems, especially when ring strain may be released during the course of reaction.

Remember from discussions in Chapter 1 that 8-membered rings are difficult to form due to internal steric hindrance in this flexible system. Cyclization reactions are unpopular, and have little success, in forming ring sizes of 8 to 12. By using a Cope rearrangement, a smaller 4-membered ring, is used as a precursor to allow entry into the generally difficult to form 8-membered ring system.
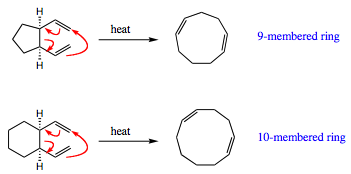
The ring systems shown above also work, although the reaction rate is not quite as quick. Five and six-membered rings do not suffer from the same ring strain issues as a 4-membered ring, so the reaction is not pushed to the same extent by ring strain. However, it is possible for these reaction to occur in fairly good yields to form 9- and 10-membered rings.
Bullvalene:
There have been many studies to determine what boundaries will help to accelerate a Cope reaction, and if a structure could be designed such that the delocalized isomer is the lowest energy possibility. Although finding this “Holy Grail” has yet to be achieved, it has led to great breakthroughs pertaining to Cope and “Cope-like” rearrangements.
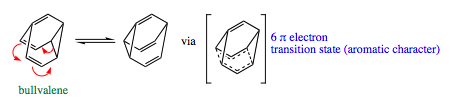
Bullvalene is popular to use as a probe to Cope rearrangements. The bridge joining the diene (shown below) may be altered to give a variety of skeletal structures with differing strain energies and connectivity. A bullvalene molecule with a lithium ion complexed to the pi-system has been hypothesized to favor a delocalized structure as shown below on the right. Although this molecule has not been isolated, it also has led to many great insights in rearrangement reactions.

Oxa-Cope (Claisen) Rearrangement
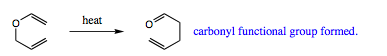
An oxa-Cope rearrangement is identical to a Cope rearrangement with the exception that an oxygen atom has replaced a carbon atom in the diene structure. This reaction is also referred to as a Claisen rearrangement. In general, an ally vinyl ether is converted, under thermal conditions, to a unsaturated carbonyl group.
A general reaction is shown below:
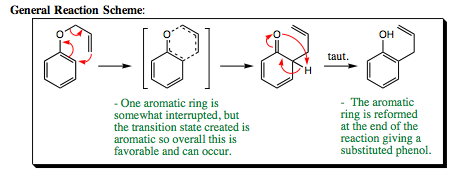
This reaction is popular with both aliphatic compounds and aromatic compounds. An example of this reaction occurring on an aromatic system is shown below:
General Reaction Scheme:
The molecular orbital picture for a Claisen rearrangement is the same that was seen with the original Cope rearrangement.
- Molecular Orbital Picture:
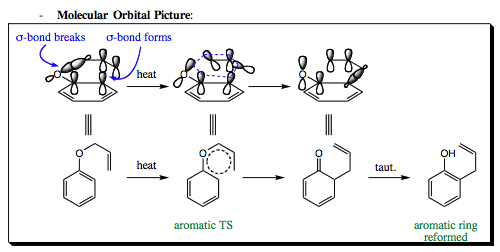
The oxa-Cope rearrangement is especially powerful when designed for use in cyclic, non-aromatic compounds. In the examples below, an allyl vinyl ether is heated and rearrangement occurs to give a bis-substituted 5-membered ring containing both a C=C and a C=O bond. If you remember from previous chapters, these functional groups will often react under similar condtions so, being able to place them side by side on a ring structure is a powerful technique indeed.
Non-Aromatic Cyclic Example:
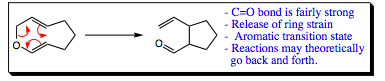
Cope/Oxa Cope summary:
1) |
Reactions go through an aromatic transition state just as was seen in Diels-Alder Reactions |
2)
|
For the oxa cope rearrangement, a carbonyl functional group is generally created from an ether functional group. (Tautomerization will occur if a ring is able to rearomatize at the end of the reaction as we witnessed). |
3)
|
Reactions may theoretically go back and forth as was the case with Diels-Alder Reactions as well. In an oxa-Cope rearrangement, the carbonyl functional group is favored and formation of this group usually drives the direction of reaction. |
| PREVIOUS PAGE (15 & 16) | Back to Index | NEXT PAGE (19 & 20) |