pp. 19 & 20
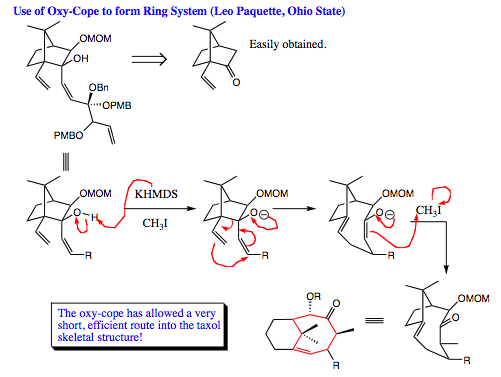
Oxy-Cope Rearrangement
Although this reaction is spelled in a very similar manner to the oxa-Cope reaction, they are quite different. The overall mechanism of an oxy-Cope is the same as that for a Cope and an oxa-Cope reaction, however, the oxy-Cope reaction has an alcohol functional group present on the diene skeletal structure. In this case the oxygen lone pair of electrons directly interacts as a part of the mechanistic pathway to help drive the reaction to completion.
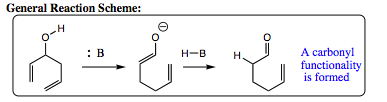
As seen above, the oxy-Cope reaction is favored to occur under basic conditions. It can go forward under neutral conditions, but the reaction must be heated for success to be realized. If the reaction is conducted under basic conditions, it can be promoted easily at room temperature and has been known to occur at temperatures as low as -78 íC. The oxy-Cope rearrangement proceeds 1017 times faster than a Cope rearrangement on a hydrocarbon system. Once again, we see the power of electron flow in organic reaction transformations.
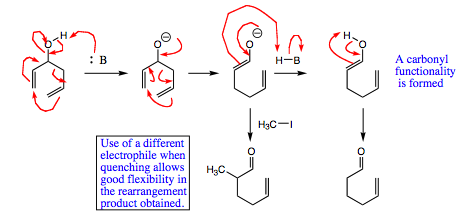
As the mechanism below shows, the base deprotonates the alcohol, giving an oxygen anion. The anion donates its lone pair into the skeletal structure, causing rearrangement and formation of an enolate. The enolate is protonated, followed and tautomerizes to give a carbonyl functional group.
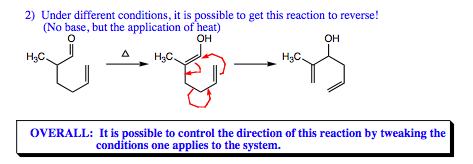
It is possible to get this reaction to go in the reverse direction as shown below, but a tremendous amount of energy is required, and yields in the reverse direction are quite low. In general, you will not see the reverse reaction utilized in an oxy-Cope reaction.
Cope/Oxa/Oxy Cope summary:
1. Reactions go through an aromatic transition state just as was seen in Diels-Alder reactions.
2. For the oxa Cope rearrangement, a carbonyl functional group is generally created from an ether functional group. (Tautomerization will occur if a ring is able to rearomatize at the end of the reaction as we witnessed).
3. Reactions may theoretically go back and forth as was the case with Diels-Alder Reactions as well.
| PREVIOUS PAGE (17 & 18) | Back to Index | NEXT PAGE (21 & 22) |