pp. 21 & 22
There are innumerable substituents that could be placed on an aromatic ring. We do not have time or space to cover all of them. What we will look at in the next few pages are how to form some of the more popular groups found on an aromatic ring, and how the chemistry of these groups may be used. Let us begin our substituent trek.
Nitration: Placing a Nitro group on the aromatic ring
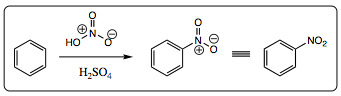
Mechanism of a nitration reaction:
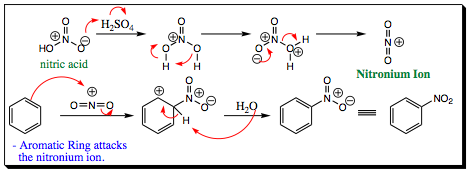
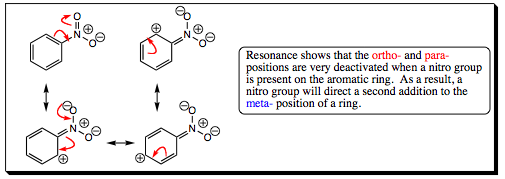
The nitronium ion is formed in situ by reaction of nitric acid with sulfuric acid. Sulfuric acid will protonate nitric acid (which acts as the base), leading to a loss of water, and formation of the nitronium ion. The nitronium ion is a very powerful electrophile and is attacked quickly by the aromatic ring. The ring is rearomatized when either water, or another nitric acid, removes a proton from the arenium ion. A nitro group is a very powerful electron withdrawing group and will direct addition of a second substituent to the meta- position. The resonance structures below show how the nitro group effects electron movement of the pi-system of an aromatic ring.
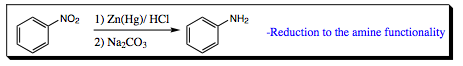
The nitro group is a very powerful synthetic tool as it not only directs meta- addition, but this functional group may be easily reduced to an amine group, which is a powerful electron donating group!!!! Reduction of the nitro group occurs under Clemmenson reduction conditions as shown below:
May reduce the nitro group to an amino group:

Once the amino group is formed, more changes are possible! An amino group may be transformed into a diazonium salt as shown below:

Diazonium salts are useful in formation of colored compounds used in dyes. One example of dye formation is shown below:
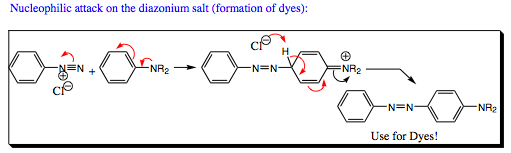
A diazonium salt is also useful to an organic chemist as it may be reduced to an alkane when heated in the presence of NaBH4.
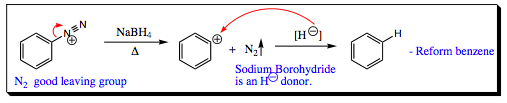
With this final reaction, it is now possible to add an EWG to an aromatic ring, reduce this group to an EDG, and finally remove it all together!!! For an organic synthetic chemist, this is a very powerful substituent for use in placing other groups onto an aromatic system.
| PREVIOUS PAGE (19 & 20) | Back to Index | NEXT PAGE (23 & 24) |