pp. 23 & 24
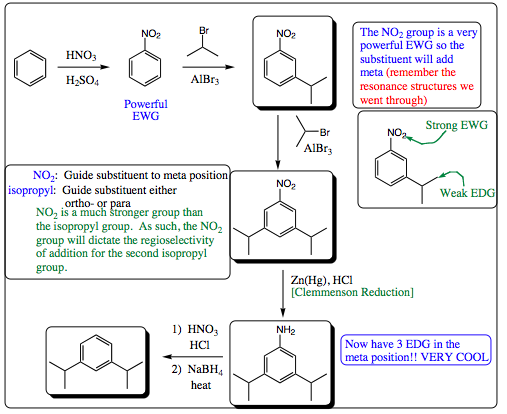
An examples of how the nitrogen atom and its various transformations may be used to control regioselectivity in EAS reactions is shown below:
Sulfonation: Fuming Sulfuric Acid (SO3 dissolved in H2SO4)
Another popular group found on aromatic rings is the sulfonyl group (think about p-toluene sulfonyl chloride used to make an alcohol a good leaving group and think about p-toluene sulfonic acid which is a very nice organic acid. Fuming sulfuric acid is so named due to the vapor that is formed when SO3 comes out of solution and reacts with water vapor to form H2SO4, which is a liquid. This formation occurs in air and gives the appearance of a "fog" although you would not wish to breathe this vapor.

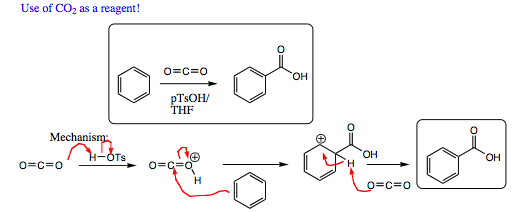
Once the substituents have been placed on an aromatic ring, all the traditional reactions you have been learning over the past 10 chapters may be conducted on the substituents (ie, Grignard, nucleophilic displacements, Williamson ether synthesis, etc.). Formation of a carboxylic acid group and subsequent transformations are shown below as an example:
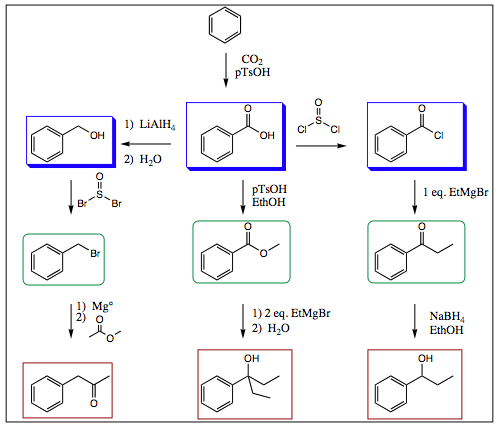
This process could be carried out indefinitely, however, these few examples illustrate how the substituents may be manipulated by traditional chemistry once they are placed on the aromatic ring itself.
| PREVIOUS PAGE (21 & 22) | Back to Index | NEXT PAGE (25 & 26) |