pp. 19 & 20
Fridel-Crafts alkylation places an EDG group onto an aromatic ring while Friedel-Crafts acylation places an EWG on the ring. If both reactions were to be conducted on an aromatic system to place two substiuent groups onto the ring, would the relative order of these two reactions help determine regioselective placement of the groups in our product? To look more closely at this question, let us view the example shown below:
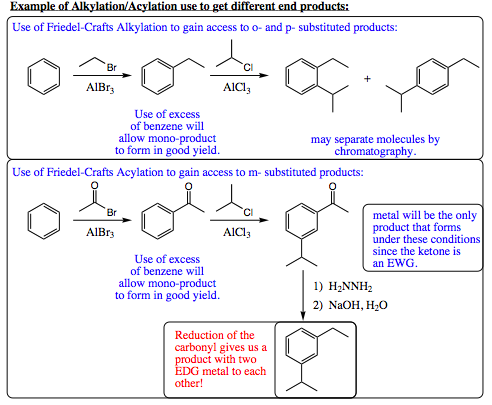
As seen from the example above, the Friedel-Crafts Alkylation and Acylation reactions are powerful synthetic tools by themselves, and are even more powerful in combination. The use of these two sets of reactions allows for a very wide diversity of aromatic molecules to be constructed efficiently, and with good control.
Regioselectivity of Halogens on Aromatic Rings:
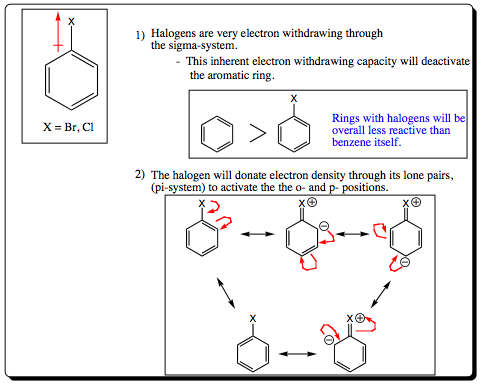
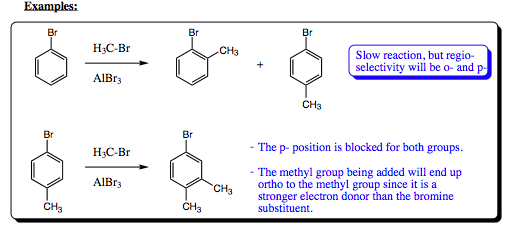
Halogen atoms constitute a bit of an enigma for the organic chemist. As we have seen in previous chapters, they are very electron withdrawing due to their electronegative properties. As we also witnessed in Chapter 9, however, the lone pairs of a halogen atom may be donated to a partially positive, or positive pi-system (remember the bromonium ion). With aromatic systems, we will see the same type of effect with halogens. They are very electron withdrawing through the sigma system and making the aromatic ring partially positive in character, and slowing the rate of an EAS. A halogen atom will donate a lone pair of electrons into the pi-system, however, activating the ortho- and para- positions of the ring. So..halogens will deactivate a ring pertaining to overall rate kinetics in the same manner as any EWG, but they will direct addition of a second group to the ortho- and para- positions in a fashion similar to an EDG. In this sense, they are a bit of an enigma.
| PREVIOUS PAGE (17 & 18) | Back to Index | NEXT PAGE (21 & 22) |