pp. 17 & 18
Specific Example of a Friedel-Crafts Acylation:
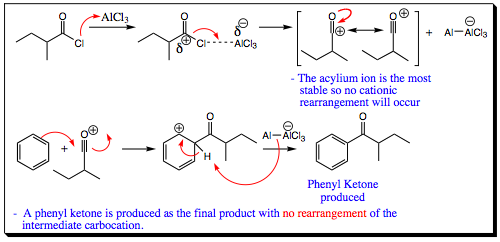
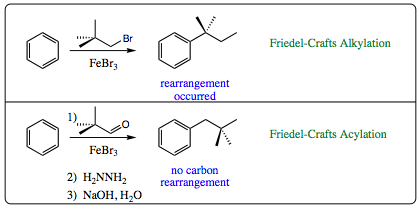
Use of the acyl group means that rearrangement does not become a problem. What about multiple addition of reagents to the ring as was witnessed in alkylation reactions?
Di -acylation?
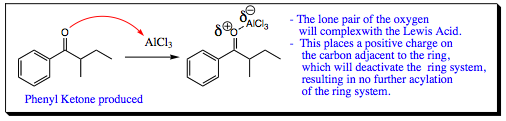
The carbonyl group (ketone) that is formed as the product is an electron withdrawing group (EWG). As such, the product formed in the reaction mixture will be less nucleophilic than the starting material and will not effectively compete with attack on electrophiles. Complexation with the Lewis acid as shown above will further deactivate the product. As such, multiple additions to the ring are generally not witnessed in a Friedel-Crafts Acylation.
These aspects of the acylation reaction, no cationic rearrangement and no multiple additions, are all very exciting, but what if the ketone is not your desired product. What if the alkyl group is truly desired? Can the Friedel-Crafts Acylation reaction be used to attach alkyl groups to aromatic rings as well as carbonyl groups? We need to go back to some older chemistry to see that it is quite easy to form an alkyl group from the phenyl ketone.
Removal of Carbonyl Carbon.
An aldehyde and ketone may be reduced to an alkane via the Wolff-Kishner Reduction (Click HERE for a review of the Wolff-Kisner reduction) as we learned in Chapter 6.
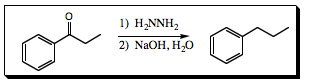
Once the phenyl ketone has been formed and isolated, mixing the product with hydrazine to form a hydrazone followed by addition of aqueous NaOH will result in formation of the alkyl group. Since these conditions are neutral (step 1) and anionic (step 2) cationc rearrangement of the alkyl group is not a concern. This method is quite popular.
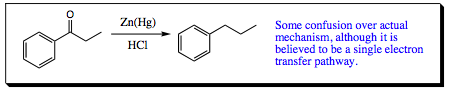
Another popular method for reducing a ketone (or aldehyde) to an alkane is the Clemmenson Reduction. The actual mechanism for this reaction is a bit cloudy so we will not worry too much about that in this text. Nevertheless, the Clemmenson Reduction is very useful and is a reaction you may wish to add to your chemical repertoire.
Clemmenson Reduction
Let us go back to our earlier alkylation example when the desired product was not isolated due to rearrangement. Use of Friedel-Craft Acylation conditions will allow for formation of the originally desired product.

We have now witnessed how substituent groups on an aromatic ring can alter pi-bond electron density present on the ring system. We have also seen how donation or withdrawal of electron density can slow (EWG) or increase (EDG) the rate of an EAS reaction. Can electron density also affect the regioselectivity of an EAS reaction. In other words, can the presence of an EDG or EWG determine the position of addition of a second group under EAS condtions? This is a question we will answer in the next few pages.
| PREVIOUS PAGE (15 & 16) | Back to Index | NEXT PAGE (19 & 20) |