pp. 33 & 34
Use of Hydrazine as a Nitrogen Nucleophile:
Nitrogen is prevalent in nature, and is popular to use in organic chemistry. As a result there are many types of nitrogen nucleophiles that have found wide use in organic chemistry systems due to their flexibility in transformations and ease of manipulation. HYDRAZINE is a popular reagent that we will spend some time studying.
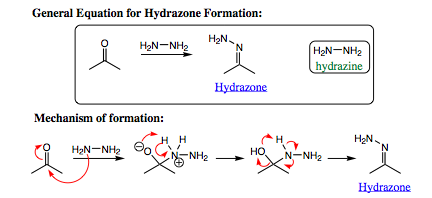
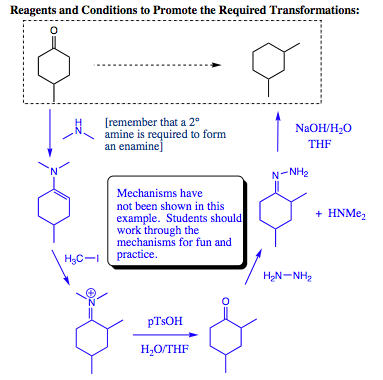
Hydrazine will add to a carbonyl compound (aldehyde or ketone) in the same fashion as witnessed for amines. The difference with hydrazine lies in the HYDRAZONE product which is formed. This product contains two nitrogen atoms and two acidic protons attached to one of the nitrogen atoms. As such, this system may be quite easily manipulated. One of the more favored reactions with this system is called the Wolff-Kishner Reduction. The Wolff-Kishner reduction entails addition of NaOH/H2O to the hydrazone. The subsequent reaction reduces the hydrazone to an alkyl group. There are not many methods for conducting this transformation, making the Wolff-Kishner reduction very powerful indeed.
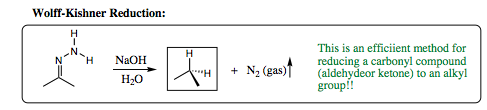
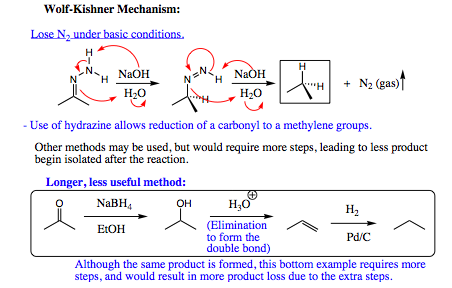
Below is an example of using enamine formation and the Wolff-Kishner reaction to obtain a desired synthetic product:
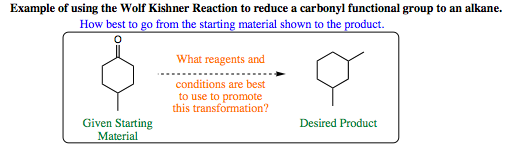
There are several theoretical routes that could work to promote the transformation above. If the route you thought up is better than the one I have proposed below, congratulations, you are honing your organic chemistry skills quite well. I have listed below a valid set of conditions for the proposed transformation. This set of reactions involves enamine formation, its use as a nucleophile, conversion back to the ketone, formation of the hydrazone, and reduction to the alkane under Wolff-Kishner conditions. Each of the steps separately is simple, yet when combined, they create an effective synthetic scheme for an organic chemist to utilize.
| PREVIOUS PAGE (31 & 32) | Back to Index | NEXT PAGE (35 & 36) |