pp. 7 & 8
The orbitals have one lobe larger than than the other. Each orbital contains one node, located at the nucleus. As was witnessed with the p-orbitals, the symmetry element reverses upon passing through the node. Hybridization creates 4 sp3-orbitals, each of which is identical to the other. This model satisfies both the Kekule and van't Hoff models put forth and allows for accurate extrapolation and prediction of organic systems. The HYBRIDIZATION MODEL is the currently excepted explanation for the bonding and structure found in carbon centers bound to four other substituents. These systems are called sp3-carbon systems. With carbon, we are concerned mainly with the sp3 orbitals since the 1s orbital is completely filled and chemically unreactive. The sp3 electrons that fill these orbitals are referred to as the valence electrons or outer shell electrons. With the electron and orbital configuration all set, how should this model now appear? We have placed electrons in our orbitals and are now dealing with electrostatic repulsion, which will push the electrons, and orbitals, spatially as far from each other, while remaining attracted to the nucleus, as
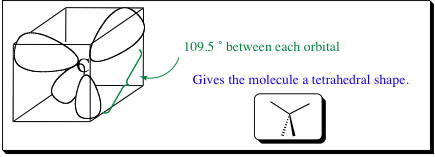
possible. With 4 identical orbitals, the 3-dimensional shape that will emerge is a tetrahedran. A tetrahedron may be best thought of as having the carbon atom inscribed in the center of a cube. Each of the orbitals then points to the farthest locations of the cube which is as distant as the sp3 electrons/orbitals may get. In this conformation, the electrons
|
|
(For a larger vesion of the movie, please click here.)
will be as spatially removed from each other as possible, minimizing electrostatic repulsion, and lowering the overall energy of the molecular configuration. We will see shortly that molecular shapes arise from the orbital and electron configuration, so it is important to know how the orbitals align around the central atom.
We will see in Chapter 2 how hybridization may also lead to trigonal planar and linear shapes discussed in the beginning of this chapter.
Bonding in sp3-Hydrocarbon Systems:
Hydrocarbon Systems:
When we look at bonding in general, the first question to ask is: "Why do atoms form bonds at all?" This may be explained using the same reasoning as to why electrons are attracted to nuclei. There is an interplay between the attractive forces of electrons with the nucleus and electrostatic repulsion between electrons. What is found is that all atoms strive to attain an Inert Gas Configuration. In each of the inert gases, the valence shell is completely filled with electrons, each electron of which is paired. For first row elements, an inert gas configuration is achieved with 2 electrons (Helium). In second row elements, an inert gas configuration is achieved with 8 electrons due to hybridization of the orbitals(Neon). If one more electron is added to an electron with an inert gas configuration, it must go into a higher energy orbital where it remains by itself in an unpaired state (Seems like a lonely existence.). If one electron is removed, there is a "hole" in the valence shell, and one of the 7 remaining electrons will also be unpaired. The best trade-off between maximizing electron attraction to the nucleus and minimizing electron repulsion is when the valence shell is completely filled. For atoms not lucky enough to be inert gases, electrons will be picked up or dropped in an attempt to gain an electron configuration that mimics an inert gas (Call it an electronic version of trying to keep up with the Jones's). Whether an atom will pick up or lose an electron depends much on where it is located in the periodic table. As the picture below illustrates, there are trends that may be followed to help determine the propensity for an atom to pick up or lose electrons.
| PREV. PAGE (5 & 6) | Back to Index | NEXT PAGE (9 & 10) |