pp. 9 & 10
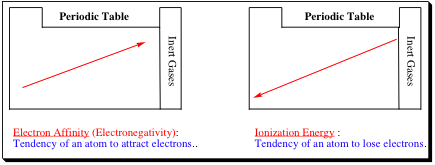
As shown above, the Electronegativity, or tendency for an atom to pick up electrons increases when traveling from left to right across a row and bottom to top in a column. Likewise, the Ionization Energy, or tendency for an atom to lose an electron increases from right to left in a row and from top to bottom in a column. If this does not make complete sense immediately, that is fine. Most great breakthroughs in chemistry were accomplished by mistake, so you are not expected to understand everything immediately. A few examples, as shown below, should clarify why the trends are seen in these particular directions. We will look at two major type of bonding, Ionic and Covalent, to help us understand these trends in better depth.
1) Ionic Bonding: Ionic bonding occurs between atoms that reside on different ends of the periodic table. In an ionic bond, there is an attraction between a very electronegative atom and a very electropositive atom. In this system electrons are not equally shared, but are transfered from the electropositive element to the electronegative element.
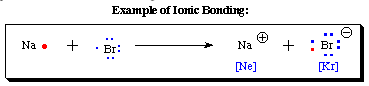
As seen in the example above, sodium (Na) has only one electron in its outer shell. If it is able to give this electron to another atom, it would then be isoelectronic to neon (an inert gas). The other element in this reaction, Br, has 7 electrons in its outer shell. If it could "find" an electron from another source, it would then have a full valence shell and would look like krypton (an inert gas). Since electrons carry a -1 charge, the sodium will carry a +1 charge after "giving" its electron to bromine and bromine will carry a -1 charge after accepting the electron. Even though the individual atoms are no longer neutral, both atoms now have "inert gas configurations" and the overall ionic complex that forms is neutral. The NaBr ionic compound formed from the reaction of sodium and bromine atoms is more stable than the two isolated atoms. The attraction between the Na cation and Br anion is called an electrostatic attraction and creates a very strong bond. Ionic complexes tend to have very high boiling points.
2) Covalent Bonding: Covalent bonding is the sharing of electrons between atoms to form a more stable molecule.
Carbon is located in the middle of a row in the periodic table with 4 electrons in its outer shell (remember the hybridization). For carbon to look like neon, it would have to pick up four electrons, thus carrying a -4 charge. To look like helium, it would have to lose 4 electrons and carry a +4 charge. Nature generally does not allow deviations from neutral of more than +2 or -2. The prospect of having to carry either a +4 or -4 charge makes Nature weep!
In 1916, G.N. Lewis proposed the concept of Covalent Bonding. If the electrons are shared between atoms, and not fully accepted or given up, then individual atoms do not have to carry high charges. Lewis's method of showing the valence electrons also introducted a more simple method of keeping track of electrons and bonds. With Lewis Dot Structures, the atom's valence shell (outer shell) electrons are shown by dots.
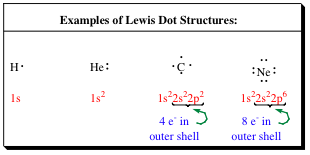
A quick glance at the Lewis dot structures for the atoms above show us that the hydrogen 1s orbital is only 1/2 filled, but that the helium 1s orbital is completely filled. As we would expect, hydrogen atoms do not exist by themselves, but helium atoms do exist as monomeric atoms. The Lewis dot structure also quickly reveals that carbon has 4 electrons in its valence shell. For carbon to look like neon, it would have to pick up 4 electrons, and to look like helium, it would have to drop 4 electrons. As we have discussed, neither of these options is something that Nature will allow. So...What can be done to stabilize hydrogen atoms and carbon atoms? One thing is to mix them together and let them share their valence electrons as shown below.
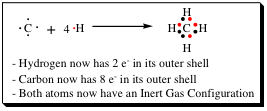
The hydrogen atom, sharing one electron with carbon, now "looks like" helium, and has achieved an inert gas configuration. The carbon, by sharing one electron with 4 different hydrogens, now "looks like" neon, and has also achieved an inert gas configuration. A very stable molecule results whenever the inert gas configuration has been achieved for ALL atoms. The beauty of Lewis Dot structures is that they may be drawn for any chemical system!! A few other carbon examples are shown below to illustrate this point.
| PREV. PAGE (7 & 8) | Back to Index | NEXT PAGE (11 & 12) |