pp. 5 & 6
Electronic Configuration of Carbon: 4-Bonds to Carbon?
All of these rules appear to work quite well and are good at locating electron spatial areas within a particular atom. However, when we look at carbon more closely, we find that these rules appear to break down rather quickly as shown below:
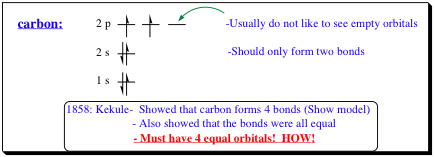
As shown, Kekule revealed in 1858 that carbon does not form only two bonds as would be predicted by its electron configuration (shown above), but is tetravalent (4 bonds). Jacobus van't Hof and Joseph LeBel further showed that a carbon bonded to 4 separate groups is tetrahedral in shape with all 4 bonds identical! The question now arises as to how this can be so when our model clearly reveals that carbon should form only two bonds and should have an empty orbital present!
(At this stage, it should be acknowledged that, since an orbital is a 90% probability of where an electron is likely to be found, there can really be no "empty" orbital as there is no probability of having an electron. For the purposes of this course, and to help visualize our compounds, we will assume that empty orbitals are plausible, and that they take up the same spatial areas as orbitals with electrons in them.)
Excitation of an Electron from a 2s to a 2p Orbital:
One method discussed to explain how 4 bonds to a carbon atom may occur is to excite an electron from the 2s to the 2p orbital:
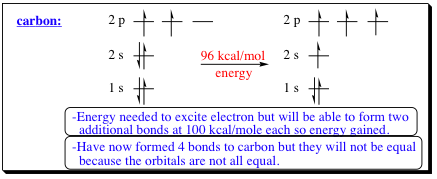
Exciting an electron from the 2s to the 2p orbital requires an initial input of energy (96 kcal/mol). A carbon-carbon bond, however, is worth approximately 100 kcal/mol. Since the carbon atom would be bonding with 4 other atoms, rather than only two, a net gain of approximately 100 kcal/mol would be recognized, making the entire process exothermic. There is a problem with this model however, and that is in the relative energies of the individual bonds forming. We know that a
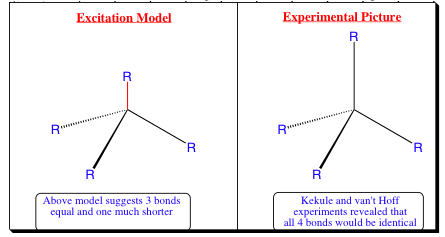
2s orbital is lower in energy than a 2p orbital. Since the described model has one 2s orbital and 3- 2p orbitals, one bond should be shorter than the other as shown in the picture below. Wxperiments have shown that all bonds are identical, so how can the Kekule and vanŐt Hoff model be explained?:
Hybridization of Orbitals (Natures Melting Pot):
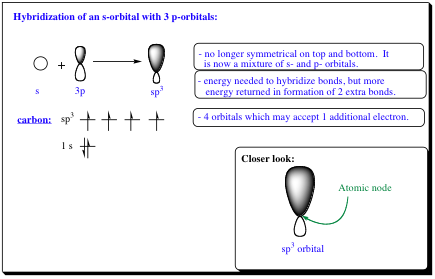
A second method that explains how 4 bonds to a carbon may occur is HYBRIDIZATION: The mixing of atomic orbitals on the same atom to form new Hybrid Orbitals.
Hybridization of orbitals does not require the input of energy that excitation of an electron from the 2s to the 2p orbital requires. In hybridization, Nature mixes one 2s orbital with three p-orbitals (4 total orbitals) to form 4-sp3 orbitals. In the picture below, it can be seen that the spherical s-orbital mixed with the symmetrical p-orbitals creates four new sp3-orbitals that are no longer symmetric.
| PREV. PAGE (3 & 4) | Back to Index | NEXT PAGE (7 & 8) |