pp. 3 & 4
The shape of an Atomic Orbital (AO) depends on the energy of the electron as denoted by the picture below:
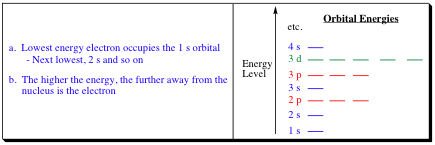
The electrons are placed into the orbitals following rules you learned in General Chemistry and which will be reviewed shortly. From previous studies, it can be determined what type of 3-D shapes for atomic orbitals correspond to the energy levels shown in the picture above. These shapes have been summarized below for the s-, p-, and d-orbital energies: The s-orbitals have the most simple geometry. They are spherical in shape and have only one symmetry sign.

The s-orbitals tend to be the lowest energy orbitals at each quantum energy level and are generally filled before the p- and d-orbitals. The p-orbitals are higher in energy than the s-orbitals at each level. The p-orbitals have one node, which is an area of zero electron density. The node for the p-orbital is located at the nucleus, meaning that the electron in this orbital will be held further away from the nucleus than an electron in the s-orbital. The p-orbital also changes symmetry sign as it passes through a node. Wherever a node is present in an orbital, the symmetry sign is reversed on either side of the node. The symmetry signs are denoted plus (+) and minus (-) but have no correlation to charge. The (+) and (-) associated with orbitals are for symmetry indications only. We will find that symmetry of orbitals plays a large part in chemical reactions, and we will need to have a good mental picture of the orbitals and their interactions to fully understand organic chemistry and the transformations that occur within these systems. The d-orbital is higher in energy than the s- and p-orbitals. The d-orbital also contains 4-nodes. The above picture shows one representation of a d-orbital and they can become quite complex. As organic chemists, we will be dealing mainly with elements in the first and second rows, which do not possess d-orbitals. As a result, we will not go into much depth pertaining to the energy and shape of d-orbitals, but you must keep in mind their presence, as they will "guide" some of the transformations we discuss.
Electronic Configuration:
As mentioned above, placing electrons into orbitals is conducted easily following a few rules you learned in General Chemistry. We will review these rules at this stage to help gain a more clear picture of the spatial aspect of the systems discussed within this course. Electrons are placed into orbitals following 3 major rules:
1) Aufbau Principle: This principle states that orbitals of lower energy are filled first. One way to remember this rule is to think of Nature as being very efficient (or lazy, whichever you wish). Why travel up 3 or 4 stairs when traveling up one stair is all that is required. When placing electrons into orbitals, it is much easier to place them in lower energy orbitals (close to the floor) than to place the electrons in higher energy orbitals (taking more steps to get further from the floor). Conserve energy whenever you can, Nature Does.
2) Pauli Exclusion Principle: Each electron must be unique as described by a unique set of Quantum numbers (4 Quantum numbers). The Principle Quantum Numbers are described as follows:
| a) | Principle Quantum Number (n): | Describes the electron enery and distance from the nucleus. |
| b) | Angular Momentum (l): | Describes the shape of an orbital. |
| c) | Magnetic Quantum Number (m): | Describes the spatial orientation and number of orbital. |
| d) | Spin Quantum Number: |
Describes the orientation of the electron with respect to an external magnetic field. The number is either +1/2 or -1/2. |
a) Principle Quantum number (n): Describes the electron energy and distance from the nucleus.
OVERALL: The first 3 numbers describe the orbital itself (ie 1s2 or 2px) while the last number describes electron spin (either spin up or spin down).
Example: H 1 electron (1s1);
He 2 electrons (1s2)
C 6 electrons (1s22s22px2py (1s22s22p2)

WHAT THE PAULI EXCLUSION PRINCIPLE FINALLY MEANS!! --- Only 2 electrons may occupy any 1 orbital and still be unique!
3) Hund's Rule: If two or more orbitals of equal energy are available, 1 electron is placed in each until all are 1/2 full.
| PREV. PAGE (1 & 2) | Back to Index | NEXT PAGE (5&6) |