Chapter 1:
Structure and Bonding in Alkanes
Organic Chemistry Revisited:
Remember that carbon is located in the middle of the 2nd row on the periodic table. With its location in the middle of the row, carbon can form a plethora of bonds with many different elements. The diverse bonding pattern leads to great diversity in compound formation, allowing carbon to integrate into some of the most important molecules and structures in the universe. Carbon, with its location in the 2nd row, is also a light element. Being relatively light enables carbon to be used in a biologically efficient manner. Try to envision if you were made up on silicon atoms (located directly beneath carbon in the periodic table). Instead of being comprised using an atom with an atomic weight of 12 g/mole, you would be comprised using an atom with an atomic weight of 28 g/mole. If you think it is difficult to get out of bed for class in the morning currently, envision weighing almost 2.5 times your present body mass!! Be happy nature picked a light element upon which to construct our mold.
The most simple organic molecules are hydrocarbons. Hydrocarbons are compounds consisting of only carbon and hydrogen atoms. Although containing only two atoms, hydrocarbons may be extremely diverse as shown with the 3 examples below:
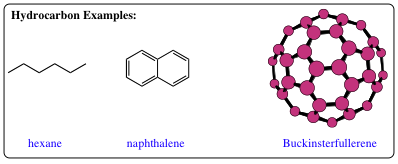
Hexane is a colorless liquid which is used often as an organic solvent and is very similar in structure to gasoline used in car engines. Naphthalene is a colorless solid which may be most noted for the smell in your grandparent's closets. Naphthalene is the smell you detect in mothballs, although it also has very intriguing chemical properties which we will be studying. Buckminsterfullerene is none other than the 3rd allotropic (pure) form of carbon. Since it contains only carbon, it is not actually a hydrocarbon, but its intrinsic beauty precludes me from leaving this compound from being mentioned within the material for too long. We will find hydrocarbons ingrained within every aspect of our lives, some examples of which are gasoline, natural gas, plastics, shampoos, etc. Thus, even though these are the most simple of organic molecules, their import and interest is more than enough to fill any Friday evening with pure conversing pleasure.
Atomic Structure:
One of the first questions to ask, and hopefully answer, is: "Why do molecules exist?" One of the most simple reasons is the favorable attraction between the negatively charged electron and the positively charged nucleus. This favorable attraction exceeds electrostatic repulsion arising from the interaction of electrons with electrons. Electrostatic forces will dominate interactions of non-bonded-atoms. Atoms connected to a central core (atom) will get as far away from each other as possible to minimize repulsive forces generated by interaction of the electron clouds. The electrostatic forces will help determine molecular shapes of molecules. Listed below are some examples of molecules and shapes that we will be studying:
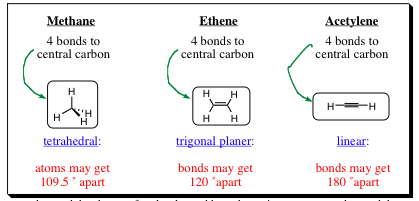
To better understand the shapes of molecules and how they arise, we must understand the atomic orbitals that help create these bonds and molecules.
Atomic Orbitals:
An atomic orbital describes the probability surface where an electron is likely to be found around a nucleus. The orbital is generally discussed at the 90-95% probability rate as trying to discuss the orbital at a 100% probability rate would have to encompass the entire size of our universe. Using the slightly lower 90-95% probability rate allows chemists to discern a very nice shape for a working model of an orbital and 3-dimensional molecular shapes.
|
|
| PREV. PAGE (Intro.) | Back to Index | NEXT PAGE (3 & 4) |