pp. 13 & 14
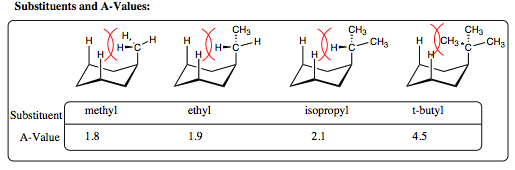
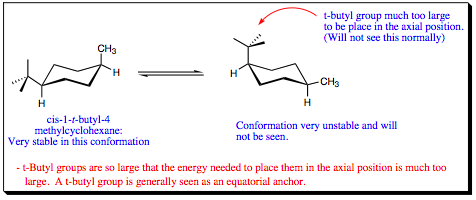
In the case of methyl, ethyl, and isopropyl groups, each of the substituents may rotate to place a hydrogen over the cyclohexane ring. As such, the amount of steric and electron repulsion witnessed in each of these systems is very similar, as reflected in the similar A-values. For the t-butyl substituent, however, all of the hydrogens have been replaced with larger methyl groups, and one methyl group must now be placed over the ring, raising the energy of the 1,3-diaxial interaction significantly. The A-value for a t-butyl group is so high that it “locks” the ring with the t-butyl substituent only in an equatorial position. A t-butyl group is never found in an axial position on a cyclohexane ring.

Having a t-butyl group “lock” the cyclohexyl ring in a particular conformation has proven useful to organic chemists over the years as an aid to studying sterics and kinetic questions pertaining to synthetic systems and other interesting phenomenon. Those experiments lie outside the scope of this class, however, if you are interested, we could all begin meeting on Sunday evenings for some more fun with cyclohexane conformers.
The amount of electron repulsion and steric hindrance will increase not just as the substituents get larger, but as more substituents are placed on the ring as shown in the movie below with cis-1,3-dimethylcyclohexane.
(For a larger version of this movie, please click here)
With two methyl groups placed in axial positions on the ring, 1,3-diaxial interactions are very high. A chair flip, in this case, will place both methyl groups in an equatorial position, which stabilizes the overall system to a great extent.
It cannot be stressed enough that you need to draw both chair conformations every time you deal with a cyclohexane system. The two examples below will show why this is so important. If you look at the left-hand picture of systems A) and B), you will note that system A) has one methyl group axial and the other methyl group equatorial. Looking then at system B), you will note that both methyl groups are axial. If these were the only conformations you drew and studied pertaining to the two systems, you would reach a conclusion that system A) is more stable overall than is system B) since system A) has only one 1,3-diaxial interaction while system B) has two such interactions. Your answer, however, would be quite incorrect. If you conduct the chair-flip and draw the other conformation in each case, you may be quite surprised. Remember that everything axial goes equatorial and vice versa during a chair flip. As such, system A) will retain one axial methyl group, and the chair flipped conformer has the same energy as the original. In system B), however, both methyl groups will reside in the equatorial position!!!! As such, the chair flipped conformer in system B) is the lowest energy conformer all 4 possibilities. System B will spend the majority of its time in this conformation and system will be the most stable overall!!! The example shown is not unusual and will hopefully serve as a reminder to always draw both chair conformations for each system to ensure that you have all the needed and required information before trying to reach a final conclusion.

| PREVIOUS PAGE (11 & 12) | Back to Index | NEXT PAGE (15 & 16) |