pp. 29 & 30
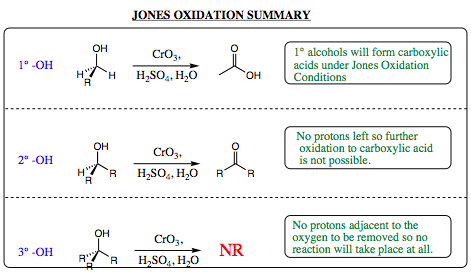
We know from Chapter 6 that 1¡, 2¡ and 3¡ alcohols have very similar chemistry. They do differ, however, in the number of R-groups attached to the carbon adjacent to the OH group. Will the differing number of R-groups have an effect on which product, if any, is formed under Jones Oxidation Conditions?

2û alcohols:
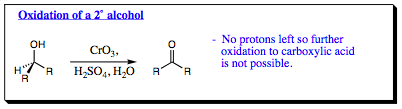
When a 2¡ alcohol is mixed with chromium trioxide under Jones Conditions, a ketone is isolated as the product. Why does the 2¡ alcohol not form a carboxylic acid as was seen with the 1¡ alcohol. A closer look at the mechanism will give us the answer to this question. As shown below, the ketone will protonate under acidic conditions and be attacked by water to form the Gem Diol (hydrate). This hydrate will react with the chromic acid complex also formed (reformed) under the acidic conditions to give a chromate ester. The chromate ester will pick up a proton, then lose a water molecule to give an unstable oxidation state of chromium. This is the exact step reached with an aldehyde. At this stage, the aldehyde proton was removed to form a carboxylic acid. With a ketone, there is no proton at the carbonyl position to be removed. Since there is no proton to remove, the reaction cannot proceed any further and will reverse all the way back to a ketone functional group upon work up and isolation. It is due to the absence of a proton on a ketone carbonyl carbon that keeps this functional group from oxidizing to a carboxylic acid. As such, 2¡ alcohols will oxidze to a ketone under Jones Oxidation conditions, but will oxidize no further.

3û alcohols

Since a 3¡ alcohol has no protons next to the oxygen, there are none that may be removed, and this functional group cannot oxidize at all. Mixing a 3¡ alcohol with chromium trioxide under Jones Oxidation conditions will yield no alcohol oxidation, and you will recover only the alcohol, or alkene products arising from elimination of water under acidic conditions, upon work up and isolation.
There are many instances when an aldehyde will be the desired product, and we will want to know how to form an aldehyde by oxidation when it is required. The methods below will reveal how this task may be accomplished.
| PREVIOUS PAGE (27 & 28) | Back to Index | NEXT PAGE (31 & 32) |