pp. 5 & 6
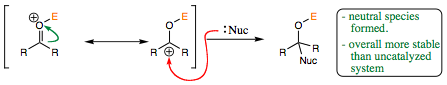
Both the catalyzed and uncatalyzed systems have been used with good success for organic transformations and bond formations. We will begin to look at which type of system works better under certain conditions. Let us begin to view these systems in more depth.
Metal Hydride Reductions (Carbonyl Reduction Reaction):
As mentioned in Chapter 3, a nucleophile is any species that may donate a lone pair of electrons. We will be reviewing many different types of popular nucleophiles in the pages to follow, however, we will start with the smallest of the nucleophilic species, the hydride.

Sodium Borohydride (NaBH4):
The hydride anion is fairly reactive and is not naturally occurring. It is most often found as a complex with a Lewis Acid type of center. We will begin with the complex of a hydride with borane to form Sodium Borohydride. Sodium borohydride is formed industrially from the reaction of sodium hydride (NaH) with timethylborate in a 4 to 1 ratio. Boron, in its neutral state, has 3 groups attached to the central
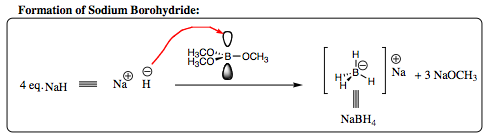
atom, and has one remaining empty p-orbital (sp2-hybridization). The hydride anion will attack the boron, overlapping with the empty orbital, placing a -1 charge on the central boron atom. The boron will “kick-out” the methoxy anion to regain neutrality, at which point another hydride anion will attack the empty orbital that has reformed. This process is repeated until 4 hydride anions are attached to the central boron atom, which now carries a negative charge.

For the central boron atom to become neutral once again, it must “kick out” a hydride anion, which will be attracted to an electrophile. Carbonyl carbons are perfect candidates!!!
Use of NaBH4 to reduce alehyde and ketone functional groups.

Addition of a hydride ion to an aldehyde or ketone will form an alcohol. This type of reaction is known as a reduction. Shown below is a general reaction of the addition of a hydride anion to either a ketone or aldehyde using sodium borohydride as the hydride source. This example assumes an aprotic solvent.

| PREVIOUS PAGE (3 & 4) | Back to Index | NEXT PAGE (7 & 8) |