pp. 3 & 4
Another reason why this first step works well is that a sigma bond is being formed to compensate for the pi-bond that has been broken. Sigma bonds have more s-character, are closer to the nucleus, and are stronger than pi-bonds. These two factors combine to make the carbonyl functional group a good electrophilic species.
The movie below shows a nice summary of a general nucleophilic attack onto a C=O bond:
(For a larger version of this movie, please click here.)
So, the success of this first step depends on how easily the pi-bond may be broken. This factor is based upon how stable the reactants are compared to the product formed from the initial nucleophile attack. There are two methods used to accelerate the first step. One method is to make the nucleophile more reactive (more negative in character). This line of thought works well to a point and many negatively charge nucleophiles are prevalent in organic synthesis. If the nucleophile becomes too reactive, we will find that it may attack not just the carbonyl functional group, but other parts of the molecule as well, giving low yields and poor product mixtures. We will pursue this line of thought a bit later. Another possibility to help promote the initial attack of the nucleophile onto the carbonyl carbon would be to make the carbonyl functional group more reactive. It would seem reasonable that if the carbonyl bond can be made more polar, the pi-bond will be weaker, and initial attack from the nucleophile will go more quickly, and more exothermically. This is in fact what does occur as shown in the system below:
Acceleration by Use of an Electrophilic Catalyst:
In many carbonyl reactions, a positively charged species is used as a catalyst to promote nucleophilic attack. Often an H+, or a metal are the catalyst of choice. An overall equation along with the energy coordinate diagram is shown below:

When an “electrophilic” catalyst is used, the first step is attack by the lone pair of electrons of the carbonyl oxygen onto the catalyst, forming a positively charged species. This step is quick and reversible (meaning that the electrophile may add and drop off a large number of times in this first reaction).
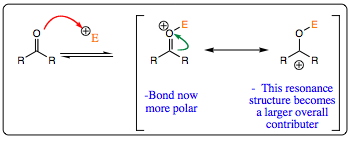
Additon of the electrophilic catalyst increases C=O bond polarity, thus increasing the partial positive character of the carbonyl carbon, accelerating attack by the nucleophile onto the carbonyl system. The intermediate formed is neutral and more stable than the anionic intermediate formed in the previous uncatalyzed system.
| PREVIOUS PAGE (1 & 2 ) | Back to Index | NEXT PAGE (5 & 6) |