pp. 37 & 38
We will find the mechanism for acidic conversion reactions very similar to base conditions, however, there will now be an extra proton “floating” in solution instead of an extra anionic species. The movie below helps to elucidate this fact for you in an animated fashion.
(For a larger version of this movie, please click here.)
Conversion of Less Reactive to More Reactive Carboxylic Acid Derivatives would, at first glance, not appear to be a possibility. You have been learning how lazy nature is, and that reactions need to form more stable products than the reactants used to form them, not the reverse. We are not going to have to break any rules, however, to find success in this endeavor. What needs to be accomplished is to take the carboxylic acid derivative we are beginning with and transform it into the carboxylate, which is the least reactive of all the derivatives.
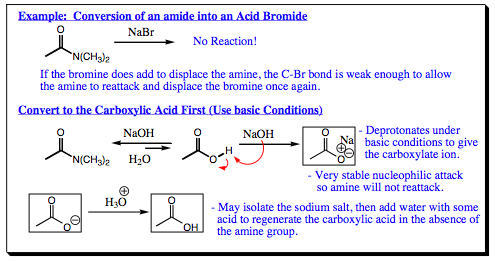
The salt of the carboxylate is most often a solid, and may be easily isolated by filtration of the reaction mixture. Once the salt has been isolated, addition of aqueous acid will regenerate the carboxylic acid. Now using a familiar reaction, we can mix the carboxylic acid with either PX3 or SOX2 to obtain an acid halide, the most reactive of all carboxylic acid derivatives!!! Although a few steps are necessary, the overall conversion from a less reactive to a more reactive carboxylic acid derivative is easily accomplished.

An example using the above set of transformations is shown below:
The beginning amide is less reactive than the ester product we wish to form. Direct conversion from an amide to an ester is generally not possible.
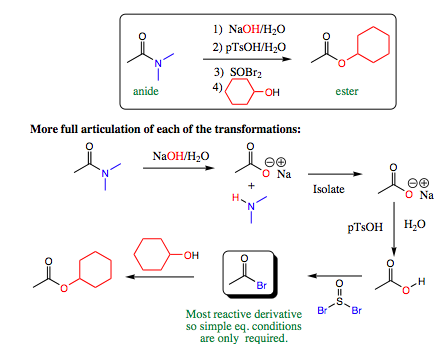
It would also be possible to form the ester from the carboxylic acid under acidic conditions. This transformation is shown below.

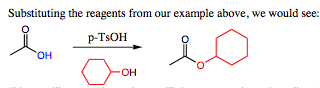
The use of base conditions will not work as a base will deprotonate the carboxylic acid, reforming the carboxylate which is unreactive.
| PREVIOUS PAGE (35 & 36) | Back to Index | NEXT PAGE (39 & 40) |