pp. 1 & 2
Chapter 2-
Introduction to Pi-Systems
In Chapter One, we dealt with molecules and structures containing a central carbon attached to 4 other groups which was determined to have an orbital hybridization of sp3. These molecules were called alkanes and cycloalkanes. Remember that carbon is a very diverse atom, and may bond to a differing number of groups ranging from one to four. The 3-dimensional structure of the molecule will be determined by the number of groups attached to the central carbon due to electrostatic repulsion pushing the attached groups as far apart as possible. The orbital hybridization will also have to be different, as nature will wish to keep the orbital energies as low as possible. Again, with four groups, the lowest energy configuration was sp3 (1 sigma orbital mixed with 3 pi-orbitals). We will study how the shape, orbital overlap, and chemistry changes when there are three and two groups attached to the central carbon.
Alkenes: Carbon Bound to Three Other Groups
Alkenes are also called olefins. In alkenes, the central carbon atom has sigma bonds to three groups rather than four groups as we witnessed with alkanes. Since bonds are formed from the overlap of orbitals, and since the number of bonds has changed, the spatial arrangement and hybridization of the orbitals will also change. Let us take a closer look at these changes.
Change in hybridization:
The formation of 3 sigma bonds requires 3 equivalent orbitals. Using the hybridization model that was discussed in the previous chapter, 1 – 2s orbital will be mixed with 2 – 2p orbitals as shown below:
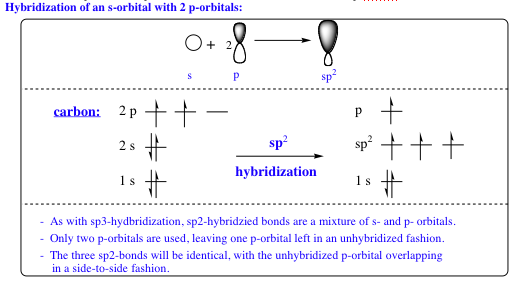
As the above figure reveals, there is one p-orbital that is not mixed, and is left unhybridized as a result. In this model, there are 3 equivalent orbitals (sp2) and 1 non-equivalent orbital (p) that are available for bonding. We can see the spatial arrangement of the orbitals themselves below:
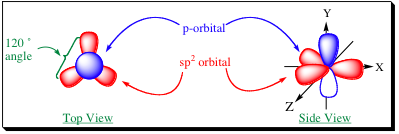
Not only is the shape of the orbitals different, but sp2-hybridized orbitals have a higher percentage of s-character (33 %) than do sp3-orbitals (25%). As a result, the electrons in an sp2-hybridized orbital will be closer to the nucleus. Having the electrons closer to the nucleus effects the bond in two major ways: 1) The bond is shorter and stronger than an sp3 bond. 2) The nucleus will have a greater attraction to the electrons resulting in a more electronegative bond than sp3-hybridized bonds.
Pi-Bonding:
The other major change in sp2-hybridized atoms arises from the unhybridized p-orbital, which has an unpaired electron and will also form a bond. Recall that sigma bonds are formed from head-to-head overlap with the electron density residing in between the two nuclei which have been joined. A p-orbital overlaps in a side-to-side fashion resulting in electron density that is above and below the two nuclei.
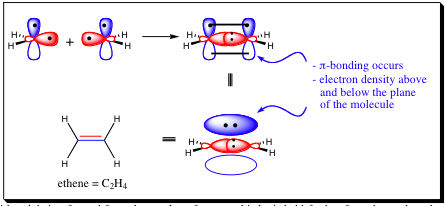
The pi-bond, being formed from the overlap of two p-orbitals, is held further from the nucleus than are the sp2 orbitals. As a result, we will find that pi-bonds may be more easily manipulated physically and chemically in many cases. Before getting too far into how we may change a pi-bond, let us first take a look at how it is formed. In the previous chapter, we learned how to form sigma bonds. It does not matter if a bond is sp2 or sp3 hybridized, the Molecular orbital diagram will be the same. Pi-bonds will form in a very similar fashion to sigma bonds as the diagram below shows, however, due to the side-to-side overlap of a pi-bond, its characteristics will be very different from that of a sigma bond.
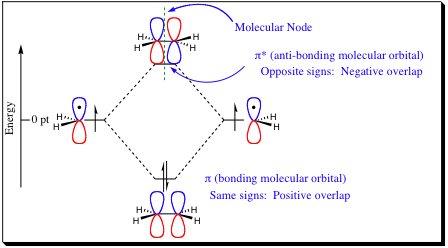
| Back to Index | NEXT PAGE (3&4) |