pp. 21 & 22
Formation of Diols:
One method to form a diol where both oxygen atoms are on adjacent carbons would be to run a substitution reaction on the newly formed hydrohalide. This reaction would be fraught with difficulty as the alcohol present would have to be protected prior to reaction, and elimination by-products could become a major sideproduct problem. Another method would be to attack an epoxide with NaOH. This method works fairly well as shown below:

There are also many possible methods for forming cis- diol structures directly from an alkene precursor. In the next few pages, we will explore these methods.
Use of Osmium Tetroxide (OsO4)- Does not overoxidize beyond the diol

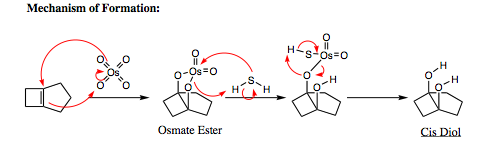
Osmium is a popular reagent because it affords high yields of the diol, and overoxidation is never a problem. Other agents may be used in Step 2 instead of H2S. The use of Na2SO3 and NaHSO3 are also popular (and a bit less smelly).
An alternative to osmium tetraoxide is potassium permanganate. The mechanism for osmium tetroxide addition and potassium permanganate addition to an alkene are almost identical!!! Potassium permanganate is more reactive than is osmium tetraoxide, which means that it may react to a greater degree and oxidize the alcohol groups further into carbonyls and carboxylic acids. Avoiding over-oxidation is accomplished by keeping the reaction temperature cold (generally 0 íC).

Potasium permanganate was historically used as an indicator for the presence of double bonds in a molecule. The oringal KMnO4 solution is dark purple. The MnO2 formed during the course of this reaction is brown and precipitates out of solution. The reaction is quite quick and helps determine if double (or triple bonds) are present within a skeletal system. With NMR now much more widely available, using KMnO4 as an indicator is not as common. As a synthetic reagent, however, it still is quite powerful.
Temperature, as mentioned above, will play a major role in this reaction! Care must be taken to ensure that you get the product you were hoping to form. To ensure diol formation, you will want to keep this reaction cold. Upon heating, further oxidation will occur to yield either a ketone or carboxylic acid.
Some examples of further oxidation are shown below:


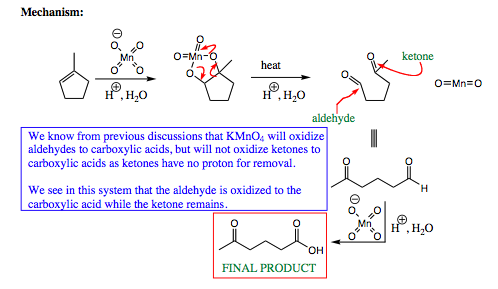
If the manganese ester complex is heated, the C-C bond may severe, as shown above, to form MnO2 and two carbonyl compounds. In the case above, an aldehyde and ketone functional group are formed. Remember from our previous discussions on oxidation (Click HERE for a review of oxidation) that KMnO4 will oxidize an aldehyde to a carboxylic acid, which is also what is witnessed above. The ketone will not oxidize further, and the final product from the alkene starting material using KMnO4 under conditions of heat is formation of an acyclic structure with a carboxylic acid and ketone functional group.
| PREVIOUS PAGE (19 & 20) | Back to Index | NEXT PAGE (23 & 24) |