pp. 25 & 26
Structure of Benzyne
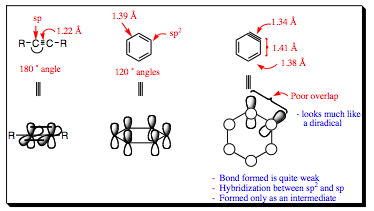
Alkyne bonds have 2 sp-hybridized carbon atoms. Remember that sp-hybridized carbon atoms have only two substituents and prefer 180¡ bond angles as a result. This may be explained by arguing that two substiuents are as far apart as possible when 180¡ from each other. It may also be explained by invoking sp-hybridization. In order to have 2-p orbitals on each carbon, the sp-orbitals must be oriented 180¡ from each other. We know from Chapters 1 and 2 that 3d-structure and hybridization are linked to each other, so both arguments would be feasible in this case. Regardless, benzyne does not allow for 180¡ angles of the carbon atoms. As shown above, C-C bond angles in benzene are held at 120¡ angles, placing a great amount of strain on the triple bond. Overlap of the alkyne orbitals is quite poor leading to a structure similar to a diradical in looks and reactivity. Benzyne structures generally cannot be isolated and are formed in situ (during the course of a reaction). We will see that they may react in a variety of ways, and we will be revisiting benzynes in later chapters, but for now, let us focus on one method of reactivity that these unique structures reveal.
Use of Benzyne:
One way in which a benzyne is used is for an easy entry into formation of phenols and aryl amines. Shown below are E1-CB conditions utilized to form phenol itself.

We will study formation of aryl halides in a later chapter, and will find they are quite readily put together. Formation of an aryl alcohol, however, generally proves to be a fair bit more difficult. One easy method to place an OH group onto an aromatic ring is via benzyne formation. In the case above, bromobenzene is mixed with NaOH at at high temperature and high pressure. The OH anion will remove a proton adjacent to the bromine (which is a good LG) forming a benzyne structure. The benzyne acts as an electrophile, being attacked by another OH anion, forming an unstabilized anion on the aromatic ring. Since OH is not a good leaving group, the anion will pick up a proton from solution instead of reforming the triple bond, given phenol as the final product.
Aryl amines may also be formed by the E1-CB mechanistic pathway. Notice that this reaction may be accomplished at a much lower temperature! Nitrogen is a much stronger base and/or nucleophile than oxygen so the first step will go much more quickly using a nitrogen anion than an oxygen anion. The final product has incorporated a nitrogen atom incorporated onto the aromatic ring instead of an oxygen, but the mechanism of formation is still identical.

Use of a strong, bulky nitrogen base will allow formation of benzyne without addition of a second equivalence of the nitrogen anion. Use of carbon anions will allow formation of benzyne and a stable alkyl side-product and are also very popular.

As a result, a second anion may be independently introduced into the system, giving a wide variety of products that now may be formed with high efficiency and control. In the following examples, a plethora of anions may be added in a second step to give nice formation of a variety of products.

Elimination of H-X from vinyl halides [Form Alkynes]
This reaction is almost identical to that just discussed with aryl systems. The big difference is in product stability. In acyclic systems, the triple bond is able to conform to 180¡ angles, giving a very stable product. As such, elimination of H-X from a vinyl system does not form just an intermediate that must react with another anion, a stable triple bond is formed!

| PREVIOUS PAGE (23 & 24) | Back to Index | NEXT PAGE (27 & 28) |