pp. 9 & 10
Irreversible Base Conditions:
Enols are always formed under acidic conditions. Acidic conditions are always reversible as the acidic proton in solution may be efficiently passed back and forth between the starting carbonyl and enol. As a result, it is not possible to form enol intermediates under irreversible conditions.
Enolates, on the other hand, may be formed under irreversible conditions, meaning that the enolate formed in solution does not revert back to starting carbonyl compound. For irreversible enolate formation to occur, a strong base must be used in the original deprotonation step. An aprotic solvent is required to ensure the enolate formed in step 1 is not able to pick up a proton from either the solvent or conjugate acid of the base used.
There are many aprotic solvents to choose from, although THF and diethyl ether tend to be the most popular for organic systems. (Click HERE for review of aprotic solvents). Likewise, there are many irreversible bases from which we could choose. Some of the more popular irreversible bases are shown below:

Under irreversible conditions, it is possible to control formation of either the Zaitsev or Hoffman enolate. The size of base chosen will dictate which of the two enolate isomers is favored. For an example of this demonstration, see below:
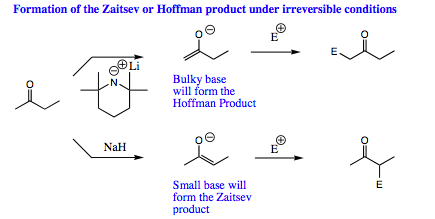
In general, a small base will form the Zaitsev enolate. A small base is able to approach either of the acidic proton sites on the carbonyl with little steric hindrance. As a result, the transition state for removal of the more hindered proton will be lower in energy as a more substituted “double bond” is being formed.
Energy of enolate formation using a small base:
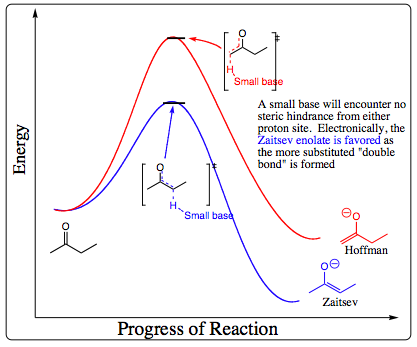
When a large, irreversible base is used, however, steric hindrance becomes an issue. A large base will be able to approach the less sterically hindered proton much more quickly to form the less hindered enolate. In this case, the less hindered proton is removed much more quickly, and the kinetic enolate is favored. The Hoffman enolate is also called the kinetic enolate as it formed faster under irreversible conditions using a big base although it is not the most stable enolate. The Hoffman enolate is not as stable as the Zaitsev, but the transition state to get to the Hoffman enolate is lower in energy than the Zaitsev enolate when a large base is used in the initial deprotonation step.
Energy of enolate formation using a large base:

We are beginning to learn how to control formation of enol and enolate intermediates so let us continue our journey by looking at a few more systems, and some of the troubles we may run into at some stage.
| PREVIOUS PAGE (7 & 8) | Back to Index | NEXT PAGE (11 & 12) |