pp. 7 & 8
Formation of Enolate and Enol species (Reversible versus Irreversible Conditions)
Reversible Condtions:
Enol Formation
Enol formation requires acidic conditions. As we mentioned previously in this Chapter, the steps in an enol formation are always reversible. What does this mean for the type of enol that will form in solution?
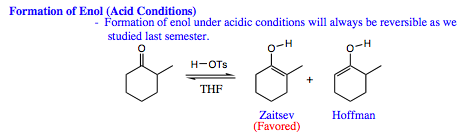
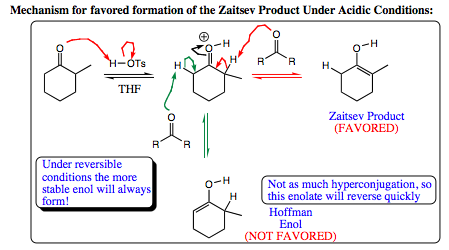
As seen above, the Zaitsev product is favored under acidic conditions. It is reasonable to believe that both enol isomers initially form in solution. The Hoffman enol may actually form more quickly as the proton at this position is less sterically hindered. Recall that the Hoffman enol is not as stable as the double bond is less substituted, thereby having fewer hperconjugative stabilizing interactions (please click HERE for review of double bond stabilization by hyperconjugation). If the Hoffman enol is less stable, it will be more likely to pick up a proton and reverse the reaction back to a carbonyl species. Once the carbonyl is reformed, both Hoffman and Zaitsev enols may form once again. If the Zaitsev isomer forms, it is more stable than the Hoffman enol, and less likely to reverse as a result.
Over time, the Zaitsev isomer will build in concentration and will be the dominant enol structure in solution (up to 97% of the ratio). As such, when an electrophile is introduced to the solution, only the Zaitsev enol is present to interact with the electrophile.
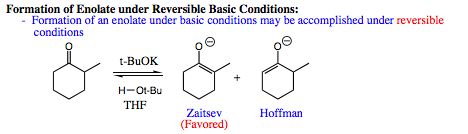
When reversible conditions are used in enolate formation under basic condtions, the Zaitsev enolate is favored in the same fashion as was witnessed under acidic conditions. The reason for this selectivity is the same as what was explained for acidic conditions. Although the proton leading to Hoffman enolate formation is less hindered and may be removed more quickly, it is not as stable and will pick up a proton from the solvent quickly to reform the starting carbonyl. When the more hindered proton is removed the Zaitsev enolate forms. The Zaitsev enolate is more stable, and will reverse to starting carbonyl much more slowly. As a result, although the Zaitsev enolate may form more slowly, it will build up in solution as the reactive intermediate.
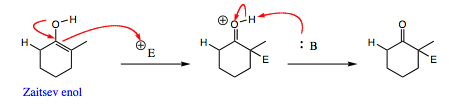
The mechanism below shows how enolates may reverse back to carbonyl starting materials:
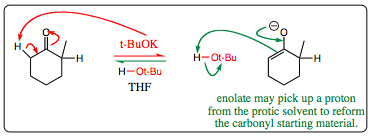
A protic solvent is necessary for this reaction to be reversible. In this case, t-BuOK is used as a base with t-BuOH used the protic solvent or co-solvent. There must be a proton source available for the enolate to receive a proton or the formation is not reversible. (For a review of protic and aprotic solvents, please click HERE)
The mechanism for enolate formation under anionic conditions is shown below. Note that a big base (t-BuOK) is used which would initially favor Hoffman enolate formation due to sterics, however, since the reaction is reversible, we can expect the more stable Zaitsev product to predominate in solution over time.
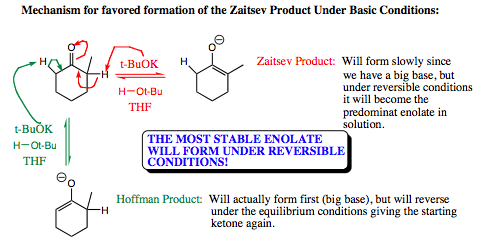
When an electrophile is introduced to the enolate mixture, the Zaitsev enolate will be prevalent (approximately 95%), and will be the enolate that reacts with the electrophilic species. A general example of this reaction is shown below:

SUMMARY OF REVERSIBLE CONDITIONS:
Use of reversible conditions under either acidic or basic conditions will result in formation of the Zaitsev enol or enolate. As a result, the electrophilic species that is introducted to the solution will “see” only the Zaitsev enol or enolate. Product formation will arise from interaction of the electrophile with the Zaitsev enol or enolate.
| PREVIOUS PAGE (5 & 6) | Back to Index | NEXT PAGE (9 & 10) |