pp. 13 & 14
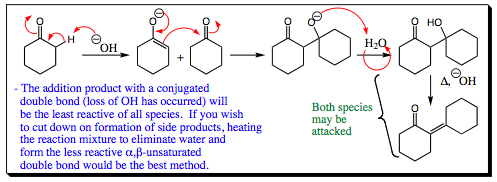
Another example of base catalyzed Aldol condensation is shown below. It is noted that both products formed under these conditions may be attacked again by an enolate, or could form an enolate themselves by reaction with base. As above, however, elimination of water to give the unsaturated carbonyl product reduces the reactivity of the carbonyl, limiting side reactions.
SUMMARY OF BASE CATALYZED ALDOL CONDENSATIONS:
Carbonyl enolates may add to themselves under the correct conditions. Side product formation is a problem in these reactions but may be minimized.
Acid Catalyzed Condensation of Carbonyls.
The Aldol condensation under acid conditions is very similar to that which was just discussed for reversible base conditions. As the mechanism below shows, unsaturated carbonyl is formed almost exclusively under these reaction conditions. Unlike reversible base conditions, the reaction under acidic conditions cannot be stopped at the alcohol stage. The carbonyl compound protonates, hydrogen bonding places a partial positive charge on the alcohol oxygen, making it a very good leaving group, and elimination is very quick.
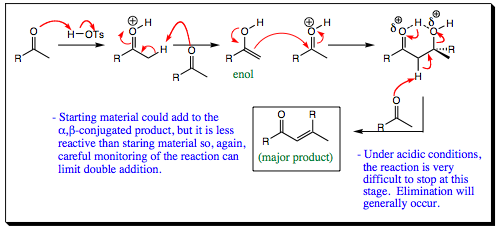
Acid catalyzed Aldol condensations are very quick and will lead almost exclusively to the unsaturated product. If this is your end goal, acid catalysis is usually the best method. Yields are quantitative in many systems. These systems are a bit limited, but if you reaction is fine running within these confines, both acid and base catalysis are very powerful synthetic methods.
In both systems shown above, it is important to note that we were dealing with symmetrical carbonyl coupling. In other words, the carbonyl was adding to itself! As mentioned before, limiting the reagents to one particular starting material cuts down vastly on side product possibilities. If two different carbonyl functional groups are used, the number of possible products increases tremendously.

In the above example, two different ketones are mixed together under reversible basic conditions. There are 6 possible products that will arise from this particular reaction. It appears to be quite a mess, and isolation of one of the products from the others would be very difficult. We would obtain the same mixture of products if this reaction were run under acidic conditions with the caveat that all of the alcohol groups would be eliminated to give an unsaturated carbonyl product.
In general, for acylic systems, reversible conditions (either acid or base) are only used when conducting a symmetrical condensation reaction. If two different carbonyl groups are required, the reaction will have to be conducted under irreversible base conditions, which will be explored in more depth in a few pages. Before we delve into irreversible base conditions, however, let us take a look at another place where reversible conditions work very well, Intramolecular Cyclization Reactions!!
Intramolecular Aldol Condensation (Robinson Annelation)
For this discussion, it will help to review ring formation as well as which rings are most stable as well and which ring sizes form the quickest (For a quick review of ring formations, please click HERE). Under reversible conditions, acid or base, the most stable molecule will form. We witnessed a preference for the most substituted enol/enolate in acyclic systems under reversible conditions, and we will witness the same preference in formation of the most stable ring structure under reversible intramolecular Aldol condensations.
Let us take a look at a very popular intramolecular Aldol condensation called the Robinson Annelation. This particular cyclization reaction is used in building the backbone to steroidal skeletal structures. A general equation is shown below:
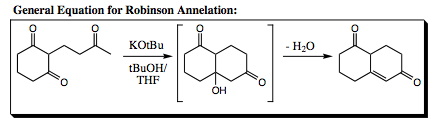
Looking at the starting material reveals a great number of acidic proton sites, all of which may react under conditions of this reaction. With all the plausible possibilities, how is it possible to form only one product, and in good yield. It does not seem possible until we begin to look more closely at the mechanism of reaction, knowing that each step is reversible and that the most thermodynamically favored (most stable) structure will be allowed to build up in solution.
| PREVIOUS PAGE (11 & 12) | Back to Index | NEXT PAGE (15 & 16) |