pp. 11 & 12
Resonance for EWG (electron withdrawing groups)
When an electron withdrawing group (EWG) is present on an aromatic ring, electron density is removed frm the pi-system, not donated. This is the opposite of what is witnessed when an EDG is present and we would expect the consequences to be opposite as well.

Once again, looking at the resonance stabilization of the intermediates as well as the electron distribution in the starting molecule should give us a clue as to why the product formation above is witnessed.
Resonance for EWG (benzaldehyde):

Using benzaldehyde as an example (the carbonyl group is electron withdrawing), it can be seen that resonance now places a positive charge within the ring. Since the aromatic ring acts as a nucleophile in EAS reactions, introducing positive character will make the ring a much less reactive nucleophile and will slow the EAS reaction considerably. Note that the positive charge is never located on the meta- position!!! When an EDG was present, the negative charge was never located on the meta- position. When an EWG is present, the positive charge is also never located on the meta- position, but only the ortho- and para- positions. We would now expect that, when an EWG group is present on an aromatic ring, the meta- position will be more reactive than either the ortho- or para-. Let us add an electrophile and see if this is, indeed, the case.
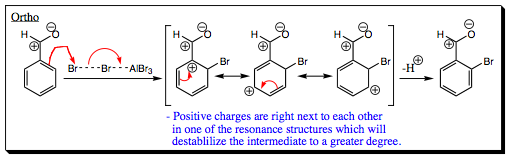
Looking at intermediates for EAS with benzaldehyde.
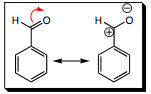
The polarity of a carbonyl group pulls the electrons toward oxygen to give the resonance structure shown above (for a review of carbonyl bond polarity, please click HERE. This is a major resonance structure and we will begin with this resonance structure in a halogenation reaction as we look at the intermediate formed in step 1.
In the first resonance structure of the arenium ion formed above, two positive centers are adjacent to each other. This is a very destabilizing resonance structure and will raise the energy of this pathway overall.
When an electrophile is added to the meta- position the positive charges are never brought next to each other.
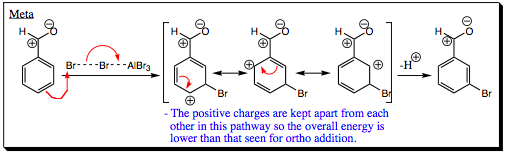
Although the meta- position is not activated when an EWG is present on the ring, it is not deactivated either. The positive charges are kept away from each other in this pathway, making it much more energetically favorable than ortho- addition.
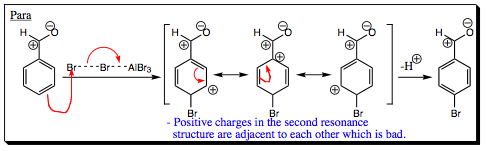
Looking at addition of an electrophile to the para- position reveals resonance character that is very similar to that shown when ortho- addition occurred (below).
Overall rate of reaction for EDG substituted aromatic rings.

Summary of EWG on an aromatic ring.
When an EWG is located on an aromatic ring, the electron density in the pi-system of the aromatic compound will be reduced with reference to an unsubstituted aromatic system. As such, an EWG substituted system is a weaker nucleophile than unsubstituted benzene and the rate of an EAS reaction with an aromatic system containing an EWG will be slowed compared to the same reaction carried out with either an unsubstittued aromatic system or an aromatic system containing an EDG.
From the resonance structures which may be drawn, it is shown the at the ortho- and para- positions are deactivated while the meta- position is not. As a consequence, aromatic systems containing an EWG will add electrophiles to the meta- position when they participate in an EAS reaction.
| PREVIOUS PAGE (9 & 10) | Back to Index | NEXT PAGE (13 & 14) |